Reciprocal regulation of Th2 and Th17 cells by PAD2-mediated citrullination
- PMID: 31723060
- PMCID: PMC6948856
- DOI: 10.1172/jci.insight.129687
Reciprocal regulation of Th2 and Th17 cells by PAD2-mediated citrullination
Abstract
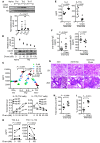
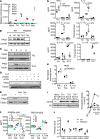
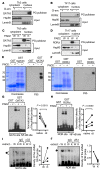
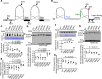
Dysregulated citrullination, a unique form of posttranslational modification catalyzed by the peptidylarginine deiminases (PADs), has been observed in several human diseases, including rheumatoid arthritis. However, the physiological roles of PADs in the immune system are still poorly understood. Here, we report that global inhibition of citrullination enhances the differentiation of type 2 helper T (Th2) cells but attenuates the differentiation of Th17 cells, thereby increasing the susceptibility to allergic airway inflammation. This effect on Th cells is due to inhibition of PAD2 but not PAD4. Mechanistically, PAD2 directly citrullinates GATA3 and RORγt, 2 key transcription factors determining the fate of differentiating Th cells. Citrullination of R330 of GATA3 weakens its DNA binding ability, whereas citrullination of 4 arginine residues of RORγt strengthens its DNA binding. Finally, PAD2-deficient mice also display altered Th2/Th17 immune response and heightened sensitivity to allergic airway inflammation. Thus, our data highlight the potential and caveat of PAD2 as a therapeutic target of Th cell-mediated diseases.
Keywords: Adaptive immunity; Allergy; Autoimmunity; Immunology; T cells.
Conflict of interest statement
Figures



Similar articles
-
The roles of PAD2- and PAD4-mediated protein citrullination catalysis in cancers.Int J Cancer. 2021 Jan 15;148(2):267-276. doi: 10.1002/ijc.33205. Epub 2020 Aug 8. Int J Cancer. 2021. PMID: 33459350 Review.
-
Citrulline Not a Major Determinant in the Recognition of Peptidylarginine Deiminase 2 and 4 by Autoantibodies in Rheumatoid Arthritis.Arthritis Rheumatol. 2020 Sep;72(9):1476-1482. doi: 10.1002/art.41276. Epub 2020 Jul 14. Arthritis Rheumatol. 2020. PMID: 32255561 Free PMC article.
-
Generation of Distinct Patterns of Rheumatoid Arthritis Autoantigens by Peptidylarginine Deiminase Types 2 and 4 During Perforin-Induced Cell Damage.Arthritis Rheumatol. 2020 Jun;72(6):912-918. doi: 10.1002/art.41196. Epub 2020 Apr 7. Arthritis Rheumatol. 2020. PMID: 31876120 Free PMC article.
-
Peptidylarginine deiminases 2 and 4 modulate innate and adaptive immune responses in TLR-7-dependent lupus.JCI Insight. 2018 Dec 6;3(23):e124729. doi: 10.1172/jci.insight.124729. JCI Insight. 2018. PMID: 30518690 Free PMC article.
-
PAD enzymes in rheumatoid arthritis: pathogenic effectors and autoimmune targets.Nat Rev Rheumatol. 2020 Jun;16(6):301-315. doi: 10.1038/s41584-020-0409-1. Epub 2020 Apr 27. Nat Rev Rheumatol. 2020. PMID: 32341463 Review.
Cited by
-
Role of the PADI family in inflammatory autoimmune diseases and cancers: A systematic review.Front Immunol. 2023 Mar 20;14:1115794. doi: 10.3389/fimmu.2023.1115794. eCollection 2023. Front Immunol. 2023. PMID: 37020554 Free PMC article. Review.
-
NETosis in the pathogenesis of acute lung injury following cutaneous chemical burns.JCI Insight. 2021 May 24;6(10):e147564. doi: 10.1172/jci.insight.147564. JCI Insight. 2021. PMID: 34027893 Free PMC article.
-
Tumor-treating fields (TTFields) induce immunogenic cell death resulting in enhanced antitumor efficacy when combined with anti-PD-1 therapy.Cancer Immunol Immunother. 2020 Jul;69(7):1191-1204. doi: 10.1007/s00262-020-02534-7. Epub 2020 Mar 6. Cancer Immunol Immunother. 2020. PMID: 32144446 Free PMC article.
-
Thinking outside the box: non-canonical targets in multiple sclerosis.Nat Rev Drug Discov. 2022 Aug;21(8):578-600. doi: 10.1038/s41573-022-00477-5. Epub 2022 Jun 6. Nat Rev Drug Discov. 2022. PMID: 35668103 Free PMC article. Review.
-
PADI4 Polymorphisms Confer Risk of Anti-CCP-Positive Rheumatoid Arthritis in Synergy With HLA-DRB1*04 and Smoking.Front Immunol. 2021 Oct 18;12:707690. doi: 10.3389/fimmu.2021.707690. eCollection 2021. Front Immunol. 2021. PMID: 34733271 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

