Interplay between degradability and integrin signaling on mesenchymal stem cell function within poly(ethylene glycol) based microporous annealed particle hydrogels
- PMID: 31711899
- PMCID: PMC6960331
- DOI: 10.1016/j.actbio.2019.11.009
Interplay between degradability and integrin signaling on mesenchymal stem cell function within poly(ethylene glycol) based microporous annealed particle hydrogels
Abstract
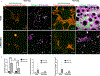
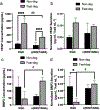
Microporous annealed particle (MAP) hydrogels are promising materials for delivering therapeutic cells. It has previously been shown that spreading and mechanosensing activation of human mesenchymal stem cells (hMSCs) incorporated in these materials can be modulated by tuning the modulus of the microgel particle building blocks. However, the effects of degradability and functionalization with different integrin-binding peptides on cellular responses has not been explored. In this work, RGDS functionalized and enzymatically degradable poly(ethylene glycol) (PEG) microgels were annealed into MAP hydrogels via thiol-ene click chemistry and photopolymerization. During cell-mediated degradation, the microgel surfaces were remodeled to wrinkles or ridges, but the scaffold integrity was maintained. Moreover, cell spreading, proliferation, and secretion of extracellular matrix proteins were significantly enhanced in faster matrix metalloproteinase degrading (KCGPQGIWGQCK) MAP hydrogels compared to non-degradable controls after 8 days of culture. We subsequently evaluated paracrine activity by hMSCs seeded in the MAP hydrogels functionalized with either RGDS or c(RRETAWA), which is specific for α5β1 integrins, and evaluated the interplay between degradability and integrin-mediated signaling. Importantly, c(RRETAWA) functionalization upregulated secretion of bone morphogenetic protein-2 overall and on a per cell basis, but this effect was critically dependent on microgel degradability. In contrast, RGDS functionalization led to higher overall vascular endothelial growth factor secretion in degradable scaffolds due to the high cell number. These results demonstrate that integrin-binding peptides can modulate hMSC behavior in PEG-based MAP hydrogels, but the results strongly depend on the susceptibility of the microgel building blocks to cell-mediated matrix remodeling. This relationship should be considered in future studies aiming to further develop these materials for stem cell delivery and tissue engineering applications. STATEMENT OF SIGNIFICANCE: Microporous annealed particle (MAP) hydrogels are attracting increasing interest for tissue repair and regeneration and have shown superior results compared to conventional hydrogels in multiple applications. Here, we studied the impact of MAP hydrogel degradability and functionalization with different integrin-binding peptides on human mesenchymal stem cells (hMSCs) that were incorporated during particle annealing. Degradability was found to improve cell growth, spreading, and extracellular matrix production regardless of the integrin-binding peptide. Moreover, in degradable MAP hydrogels the integrin-binding peptide c(RRETAWA) was found to increase osteogenic protein expression by hMSCs compared to RGDS-functionalized MAP hydrogels. These results have important implications for the development of a MAP hydrogel-based hMSC delivery system for bone tissue engineering.
Keywords: Cell-material interactions; Human mesenchymal stem cells; Hydrogel; Integrin; Matrix remodeling; Poly(ethylene glycol).
Copyright © 2019 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



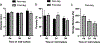

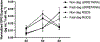
Similar articles
-
Microporous annealed particle hydrogel stiffness, void space size, and adhesion properties impact cell proliferation, cell spreading, and gene transfer.Acta Biomater. 2019 Aug;94:160-172. doi: 10.1016/j.actbio.2019.02.054. Epub 2019 May 30. Acta Biomater. 2019. PMID: 31154058 Free PMC article.
-
Enhanced articular cartilage by human mesenchymal stem cells in enzymatically mediated transiently RGDS-functionalized collagen-mimetic hydrogels.Acta Biomater. 2017 Mar 15;51:75-88. doi: 10.1016/j.actbio.2017.01.028. Epub 2017 Jan 10. Acta Biomater. 2017. PMID: 28087486 Free PMC article.
-
Osteogenic differentiation of human mesenchymal stem cells on α5 integrin binding peptide hydrogels is dependent on substrate elasticity.Biomater Sci. 2014 Mar 1;2(3):352-361. doi: 10.1039/C3BM60149H. Biomater Sci. 2014. PMID: 24660057 Free PMC article.
-
Granular Hydrogels for Harnessing the Immune Response.Adv Healthc Mater. 2024 Oct;13(25):e2303005. doi: 10.1002/adhm.202303005. Epub 2024 Jan 7. Adv Healthc Mater. 2024. PMID: 38145369 Review.
-
Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity.Signal Transduct Target Ther. 2021 Dec 16;6(1):426. doi: 10.1038/s41392-021-00830-x. Signal Transduct Target Ther. 2021. PMID: 34916490 Free PMC article. Review.
Cited by
-
The Role of Integrin Receptor's α and β Subunits of Mouse Mesenchymal Stem Cells on the Interaction of Marine-Derived Blacktip Reef Shark (Carcharhinus melanopterus) Skin Collagen.Int J Mol Sci. 2023 May 23;24(11):9110. doi: 10.3390/ijms24119110. Int J Mol Sci. 2023. PMID: 37298062 Free PMC article.
-
Hydrogel Drug Delivery Systems for Bone Regeneration.Pharmaceutics. 2023 Apr 25;15(5):1334. doi: 10.3390/pharmaceutics15051334. Pharmaceutics. 2023. PMID: 37242576 Free PMC article. Review.
-
Hydrogels to Support Transplantation of Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells.Brain Sci. 2022 Nov 25;12(12):1620. doi: 10.3390/brainsci12121620. Brain Sci. 2022. PMID: 36552081 Free PMC article.
-
Clickable Granular Hydrogel Scaffolds for Delivery of Neural Progenitor Cells to Sites of Spinal Cord Injury.Adv Healthc Mater. 2024 Oct;13(25):e2303912. doi: 10.1002/adhm.202303912. Epub 2024 Mar 24. Adv Healthc Mater. 2024. PMID: 38470994
-
Anisotropic Rod-Shaped Particles Influence Injectable Granular Hydrogel Properties and Cell Invasion.Adv Mater. 2022 Mar;34(12):e2109194. doi: 10.1002/adma.202109194. Epub 2022 Jan 24. Adv Mater. 2022. PMID: 34932833 Free PMC article.
References
-
- Burdick JA, Mauck RL, Gerecht S, To serve and protect: hydrogels to improve stem cell-based therapies, Cell stem cell 18(1) (2016) 13–15. - PubMed
-
- Wang C, Varshney RR, Wang D-A, Therapeutic cell delivery and fate control in hydrogels and hydrogel hybrids, Advanced drug delivery reviews 62(7–8) (2010) 699–710. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

