Extensive brainstem infiltration, not mass effect, is a common feature of end-stage cerebral glioblastomas
- PMID: 31711239
- PMCID: PMC7158646
- DOI: 10.1093/neuonc/noz216
Extensive brainstem infiltration, not mass effect, is a common feature of end-stage cerebral glioblastomas
Erratum in
-
Corrigendum to Extensive brainstem infiltration, not mass effect, is a common feature of end-stage cerebral glioblastomas.Neuro Oncol. 2021 Aug 2;23(8):1412. doi: 10.1093/neuonc/noaa005. Neuro Oncol. 2021. PMID: 32068875 Free PMC article. No abstract available.
Abstract
Background: Progress in extending the survival of glioblastoma (GBM) patients has been slow. A better understanding of why patient survival remains poor is critical to developing new strategies. Postmortem studies on GBM can shed light on why patients are dying.
Methods: The brains of 33 GBM patients were autopsied and examined for gross and microscopic abnormalities. Clinical-pathologic correlations were accomplished through detailed chart reviews. Data were compared with older published autopsy GBM studies that predated newer treatment strategies, such as more extensive surgical resection and adjuvant temozolomide.
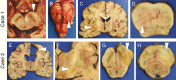
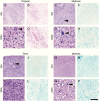
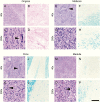
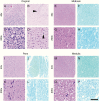
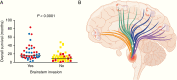
Results: In older GBM autopsy series, mass effect was observed in 72% of brains, with herniation in 50% of all cases. Infiltration of tumor into the brainstem was noted in only 21% of those older cases. In the current series, only 10 of 33 (30%) GBMs showed mass effect (P = 0.0003), and only 1 (3%) showed herniation (P < 0.0001). However, extensive GBM infiltration of the brainstem was present in 22 cases (67%, P < 0.0001), with accompanying destruction of the pons and white matter tracts. There was a direct correlation between longer median patient survival and the presence of brainstem infiltration (16.1 mo in brainstem-invaded cases vs 9.0 mo in cases lacking extensive brainstem involvement; P = 0.0003).
Conclusions: With improving care, severe mass effect appears to be less common in GBM patients today, whereas dissemination, including life-threatening brainstem invasion, is now more pronounced. This has major implications regarding preclinical GBM models, as well as the design of clinical trials aimed at further improving patient survival.
Keywords: autopsy; brainstem; glioblastoma; medulla; midbrain; pons; postmortem.
© The Author(s) 2019. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
Comment in
-
Letter regarding "Extensive brainstem infiltration, not mass effect, is a common feature of end-stage cerebral glioblastomas".Neuro Oncol. 2020 Dec 18;22(12):1882-1883. doi: 10.1093/neuonc/noaa186. Neuro Oncol. 2020. PMID: 32770191 Free PMC article. No abstract available.
Similar articles
-
Letter regarding "Extensive brainstem infiltration, not mass effect, is a common feature of end-stage cerebral glioblastomas".Neuro Oncol. 2020 Dec 18;22(12):1882-1883. doi: 10.1093/neuonc/noaa186. Neuro Oncol. 2020. PMID: 32770191 Free PMC article. No abstract available.
-
Selection of eligible patients with supratentorial glioblastoma multiforme for gross total resection.J Neurooncol. 2001 Apr;52(2):161-71. doi: 10.1023/a:1010624504311. J Neurooncol. 2001. PMID: 11508816 Clinical Trial.
-
Massive dissemination of adult glioblastomas.Clin Neuropathol. 2015 Nov-Dec;34(6):330-42. doi: 10.5414/NP300882. Clin Neuropathol. 2015. PMID: 26308254
-
The cause of death in patients with glioblastoma is multifactorial: clinical factors and autopsy findings in 117 cases of supratentorial glioblastoma in adults.J Neurooncol. 1991 Apr;10(2):179-85. doi: 10.1007/BF00146880. J Neurooncol. 1991. PMID: 1654403 Review.
-
A systematic review of overall survival in pediatric primary glioblastoma multiforme of the spinal cord.J Neurosurg Pediatr. 2017 Feb;19(2):239-248. doi: 10.3171/2016.8.PEDS1631. Epub 2016 Nov 4. J Neurosurg Pediatr. 2017. PMID: 27813458 Review.
Cited by
-
Letter regarding "Extensive brainstem infiltration, not mass effect, is a common feature of end-stage cerebral glioblastomas".Neuro Oncol. 2020 Dec 18;22(12):1882-1883. doi: 10.1093/neuonc/noaa186. Neuro Oncol. 2020. PMID: 32770191 Free PMC article. No abstract available.
-
Inference of glioblastoma migration and proliferation rates using single time-point images.Commun Biol. 2023 Apr 13;6(1):402. doi: 10.1038/s42003-023-04750-0. Commun Biol. 2023. PMID: 37055469 Free PMC article.
-
Tumor-associated alterations in white matter connectivity have prognostic significance in MGMT-unmethylated glioblastoma.J Neurooncol. 2022 Jul;158(3):331-339. doi: 10.1007/s11060-022-04018-3. Epub 2022 May 7. J Neurooncol. 2022. PMID: 35525907
-
Tumor Microenvironment and Microvascular Density in Human Glioblastoma.Cells. 2022 Dec 20;12(1):11. doi: 10.3390/cells12010011. Cells. 2022. PMID: 36611806 Free PMC article.
-
Radical surgical resection with molecular margins is associated with improved survival in IDH wild-type glioblastoma.Neuro Oncol. 2024 Sep 5;26(9):1660-1669. doi: 10.1093/neuonc/noae073. Neuro Oncol. 2024. PMID: 38581292
References
-
- Hegi ME, Diserens AC, Gorlia T, et al. . MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. - PubMed
-
- Stupp R, Wong ET, Kanner AA, et al. . NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer. 2012;48(14):2192–2202. - PubMed
-
- Giangaspero F, Burger PC. Correlations between cytologic composition and biologic behavior in the glioblastoma multiforme. A postmortem study of 50 cases. Cancer. 1983;52(12):2320–2333. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

