Geriatric Nutritional Risk Index as a prognostic marker in patients with extensive-stage disease small cell lung cancer: Results from a randomized controlled trial
- PMID: 31707767
- PMCID: PMC6938749
- DOI: 10.1111/1759-7714.13229
Geriatric Nutritional Risk Index as a prognostic marker in patients with extensive-stage disease small cell lung cancer: Results from a randomized controlled trial
Abstract
Background: Clinical impact of the Geriatric Nutritional Risk Index (GNRI) in patients with extensive-stage disease small cell lung cancer (ED-SCLC) have not previously been reported.
Methods: This study analyzed 352 patients enrolled in a previous randomized phase III trial comparing the efficacy of irinotecan plus cisplatin with that of etoposide plus cisplatin as the first-line therapy for ED-SCLC. GNRI values were calculated using serum albumin levels and actual and ideal bodyweights. Patients with a GNRI > 98, 92-98, and <92 were grouped into no, low, and moderate/major risk groups, respectively.
Results: The objective response rates were 63.2%, 52.6%, and 49.2% in the no, low, and moderate/major risk groups, respectively (P = 0.024). The median progression-free survival (PFS) was shorter in patients with a lower GNRI than in those with a higher GNRI (no vs. low vs. moderate/major risk group; 6.5 vs. 5.8 vs. 5.9 months, respectively; P = 0.028). There were significant differences in median overall survival (OS) according to GNRI (no vs. low vs. moderate/major risk group; 13.2 vs. 10.3 vs. 8.4 months, respectively; P < 0.001). Multivariate analysis revealed that being in the moderate/major risk group was an independent poor prognostic factor for PFS (hazard ratio [HR]: 1.300, 95% confidence interval [CI]: 1.012-1.670; P = 0.040) and OS (HR: 1.539; 95% CI: 1.069-2.216; P = 0.020).
Conclusions: This prospective study shows that a low GNRI value was associated with a poor prognosis, and it supports the relationship between systemic inflammation, nutritional status, and clinical outcomes in patients with ED-SCLC.Key points SIGNIFICANT FINDINGS OF THE STUDY: The lower GNRI group had a low response rate to chemotherapy for ED-SCLC. The HRs for PFS and OS were 1.300 and 1.539 in the patients with GNRI < 92.
What this study adds: Low GNRI is associated with poor prognosis in ED-SCLC.
Keywords: Cachexia; inflammation; nutrition assessment; small cell lung carcinoma.
© 2019 The Authors. Thoracic Cancer published by China Lung Oncology Group and John Wiley & Sons Australia, Ltd.
Figures
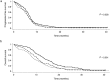
 ) GNRI > 98 (n = 133), (
) GNRI > 98 (n = 133), ( ) GNRI 92–98 (n = 95) and (
) GNRI 92–98 (n = 95) and ( ) GNRI < 92 (n = 124).
) GNRI < 92 (n = 124).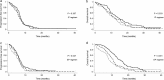
 ) GNRI > 98 (n = 63), (
) GNRI > 98 (n = 63), ( ) GNRI 92–98 (n = 47) and (
) GNRI 92–98 (n = 47) and ( ) GNRI < 92 (n = 60). (c, d) (
) GNRI < 92 (n = 60). (c, d) ( ) GNRI > 98 (n = 70), (
) GNRI > 98 (n = 70), ( ) GNRI 92–98 (n = 48) and (
) GNRI 92–98 (n = 48) and ( ) GNRI < 92 (n = 64).
) GNRI < 92 (n = 64).Similar articles
-
Association of the Geriatric Nutritional Risk Index with the survival of patients with non-small-cell lung cancer after platinum-based chemotherapy.BMC Pulm Med. 2021 Dec 11;21(1):409. doi: 10.1186/s12890-021-01782-2. BMC Pulm Med. 2021. PMID: 34895201 Free PMC article.
-
Baseline quality of life and performance status as prognostic factors in patients with extensive-stage disease small cell lung cancer treated with pemetrexed plus carboplatin vs. etoposide plus carboplatin.Lung Cancer. 2012 Dec;78(3):276-81. doi: 10.1016/j.lungcan.2012.09.002. Epub 2012 Oct 6. Lung Cancer. 2012. PMID: 23043970
-
Prognostic Value of the Geriatric Nutritional Risk Index in Previously Untreated Patients with Advanced Non-Small Cell Lung Cancer Treated with a Combination Therapy of Anti-PD-1/-PD-L1 Antibodies and Platinum-Based Chemotherapy: A Multicenter Retrospective Study.Oncology. 2024;102(10):819-827. doi: 10.1159/000536120. Epub 2024 Feb 6. Oncology. 2024. PMID: 38320539
-
Efficacy of the Geriatric Nutritional Risk Index for Predicting Overall Survival in Patients with Head and Neck Cancer: A Meta-Analysis.Nutrients. 2023 Oct 12;15(20):4348. doi: 10.3390/nu15204348. Nutrients. 2023. PMID: 37892423 Free PMC article. Review.
-
Prognostic utility of the geriatric nutritional risk index for head and neck cancer: Systematic review and meta-analysis.Head Neck. 2024 Aug;46(8):2086-2097. doi: 10.1002/hed.27842. Epub 2024 Jun 9. Head Neck. 2024. PMID: 38853422 Review.
Cited by
-
GNRI And Conut Scores: Simple Predictors of Sarcopenia in Metastatic Colorectal Cancer Patients.Support Care Cancer. 2022 Oct;30(10):7845-7852. doi: 10.1007/s00520-022-07218-9. Epub 2022 Jun 18. Support Care Cancer. 2022. PMID: 35716261
-
Geriatric nutritional risk index as an independent prognostic factor in locally advanced nasopharyngeal carcinoma treated using radical concurrent chemoradiotherapy: a retrospective cohort study.Ann Transl Med. 2021 Apr;9(7):532. doi: 10.21037/atm-20-6493. Ann Transl Med. 2021. PMID: 33987230 Free PMC article.
-
Low geriatric nutritional risk index as a poor prognostic biomarker for immune checkpoint inhibitor treatment in solid cancer.Front Nutr. 2023 Nov 1;10:1286583. doi: 10.3389/fnut.2023.1286583. eCollection 2023. Front Nutr. 2023. PMID: 38024341 Free PMC article.
-
Geriatric Nutritional Risk Index as Prognostic Marker for Elderly Patients With Small Cell Lung Cancer.Cancer Diagn Progn. 2024 Jul 3;4(4):482-488. doi: 10.21873/cdp.10352. eCollection 2024 Jul-Aug. Cancer Diagn Progn. 2024. PMID: 38962547 Free PMC article.
-
The Geriatric Nutritional Risk Index Predicts Prognosis in Japanese Patients with LATITUDE High-Risk Metastatic Hormone-Sensitive Prostate Cancer: A Multi-Center Study.Cancers (Basel). 2023 Nov 8;15(22):5333. doi: 10.3390/cancers15225333. Cancers (Basel). 2023. PMID: 38001593 Free PMC article.
References
-
- van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small‐cell lung cancer. Lancet 2011; 378: 1741–55. - PubMed
-
- Tiseo M, Boni L, Ambrosio F et al Italian, multicenter, phase III, randomized study of cisplatin plus etoposide with or without bevacizumab as first‐line treatment in extensive‐disease small‐cell lung cancer: The GOIRC‐AIFA FARM6PMFJM trial. J Clin Oncol 2017; 35: 1281–7. - PubMed
-
- Rossi A, Di Maio M, Chiodini P et al Carboplatin‐ or cisplatin‐based chemotherapy in first‐line treatment of small‐cell lung cancer: The COCIS meta‐analysis of individual patient data. J Clin Oncol 2012; 30: 1692–8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

