Antibacterial activity of human mesenchymal stem cells mediated directly by constitutively secreted factors and indirectly by activation of innate immune effector cells
- PMID: 31702119
- PMCID: PMC6988770
- DOI: 10.1002/sctm.19-0092
Antibacterial activity of human mesenchymal stem cells mediated directly by constitutively secreted factors and indirectly by activation of innate immune effector cells
Abstract
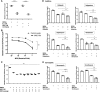
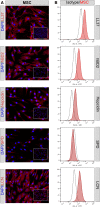
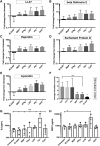
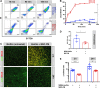
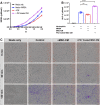
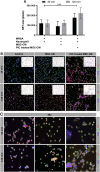
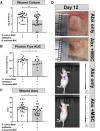
Mesenchymal stem cells (MSC) have been shown to improve wound healing and suppress inflammatory immune responses. Newer research also indicates that MSC exhibit antimicrobial activity, although the mechanisms underlying this activity have not been fully elucidated. Therefore, we conducted in vitro and in vivo studies to examine the ability of resting and activated MSC to kill bacteria, including multidrug resistant strains. We investigated direct bacterial killing mechanisms and the interaction of MSC with host innate immune responses to infection. In addition, the activity of MSC against chronic bacterial infections was investigated in a mouse biofilm infection model. We found that MSC exhibited high levels of spontaneous direct bactericidal activity in vitro. Moreover, soluble factors secreted by MSC inhibited Staphylococcus aureus biofilm formation in vitro and disrupted the growth of established biofilms. Secreted factors from MSC also elicited synergistic killing of drug-resistant bacteria when combined with several major classes of antibiotics. Other studies demonstrated interactions of activated MSC with host innate immune responses, including triggering of neutrophil extracellular trap formation and increased phagocytosis of bacteria. Finally, activated MSC administered systemically to mice with established S. aureus biofilm infections significantly reduced bacterial numbers at the wound site and improved wound healing when combined with antibiotic therapy. These results indicate that MSC generate multiple direct and indirect, immunologically mediated antimicrobial activities that combine to help eliminate chronic bacterial infections when the cells are administered therapeutically.
Keywords: antibacterial; cytokines; infection; neutrophil; peptides; stem cells.
© 2019 The Authors. Stem Cells Translational Medicine published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Conflict of interest statement
S.D. declared patent ownership on a patent covering antimicrobial stem cell technology. All of the other authors declared no potential conflict of interest.
Figures
Similar articles
-
Activated Mesenchymal Stromal Cell Therapy for Treatment of Multi-Drug Resistant Bacterial Infections in Dogs.Front Vet Sci. 2022 Jun 23;9:925701. doi: 10.3389/fvets.2022.925701. eCollection 2022. Front Vet Sci. 2022. PMID: 35812842 Free PMC article.
-
Activated Mesenchymal Stem Cells Interact with Antibiotics and Host Innate Immune Responses to Control Chronic Bacterial Infections.Sci Rep. 2017 Aug 29;7(1):9575. doi: 10.1038/s41598-017-08311-4. Sci Rep. 2017. PMID: 28851894 Free PMC article.
-
Mesenchymal stromal cell-secreted CCL2 promotes antibacterial defense mechanisms through increased antimicrobial peptide expression in keratinocytes.Stem Cells Transl Med. 2021 Dec;10(12):1666-1679. doi: 10.1002/sctm.21-0058. Epub 2021 Sep 16. Stem Cells Transl Med. 2021. PMID: 34528765 Free PMC article.
-
Neutrophils in innate host defense against Staphylococcus aureus infections.Semin Immunopathol. 2012 Mar;34(2):237-59. doi: 10.1007/s00281-011-0295-3. Epub 2011 Nov 12. Semin Immunopathol. 2012. PMID: 22080185 Free PMC article. Review.
-
Epic Immune Battles of History: Neutrophils vs. Staphylococcus aureus.Front Cell Infect Microbiol. 2017 Jun 30;7:286. doi: 10.3389/fcimb.2017.00286. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28713774 Free PMC article. Review.
Cited by
-
Preliminary evaluation of safety and migration of immune activated mesenchymal stromal cells administered by subconjunctival injection for equine recurrent uveitis.Front Vet Sci. 2023 Dec 14;10:1293199. doi: 10.3389/fvets.2023.1293199. eCollection 2023. Front Vet Sci. 2023. PMID: 38162475 Free PMC article.
-
Activated Mesenchymal Stromal Cell Therapy for Treatment of Multi-Drug Resistant Bacterial Infections in Dogs.Front Vet Sci. 2022 Jun 23;9:925701. doi: 10.3389/fvets.2022.925701. eCollection 2022. Front Vet Sci. 2022. PMID: 35812842 Free PMC article.
-
Impact of Three Different Serum Sources on Functional Properties of Equine Mesenchymal Stromal Cells.Front Vet Sci. 2021 Apr 30;8:634064. doi: 10.3389/fvets.2021.634064. eCollection 2021. Front Vet Sci. 2021. PMID: 33996964 Free PMC article.
-
Immune Activated Cellular Therapy for Drug Resistant Infections: Rationale, Mechanisms, and Implications for Veterinary Medicine.Vet Sci. 2022 Nov 4;9(11):610. doi: 10.3390/vetsci9110610. Vet Sci. 2022. PMID: 36356087 Free PMC article. Review.
-
Stem Cell-Derived Exosome as Potential Therapeutics for Microbial Diseases.Front Microbiol. 2022 Feb 14;12:786111. doi: 10.3389/fmicb.2021.786111. eCollection 2021. Front Microbiol. 2022. PMID: 35237239 Free PMC article. Review.
References
-
- Arciola CR, Campoccia D, Montanaro L. Implant infections: adhesion, biofilm formation and immune evasion. Nat Rev Microbiol. 2018;16:397‐409. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

