The exosome-mediated autocrine and paracrine actions of plasma gelsolin in ovarian cancer chemoresistance
- PMID: 31700155
- PMCID: PMC7018662
- DOI: 10.1038/s41388-019-1087-9
The exosome-mediated autocrine and paracrine actions of plasma gelsolin in ovarian cancer chemoresistance
Abstract
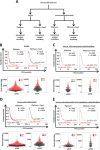
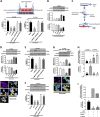
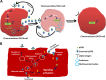
Ovarian cancer (OVCA) is the most lethal gynecological cancer, due predominantly to late presentation, high recurrence rate and common chemoresistance development. The expression of the actin-associated protein cytosolic gelsolin (GSN) regulates the gynecological cancer cell fate resulting in dysregulation in chemosensitivity. In this study, we report that elevated expression of plasma gelsolin (pGSN), a secreted isoform of GSN and expressed from the same GSN gene, correlates with poorer overall survival and relapse-free survival in patients with OVCA. In addition, it is highly expressed and secreted in chemoresistant OVCA cells than its chemosensitive counterparts. pGSN, secreted and transported via exosomes (Ex-pGSN), upregulates HIF1α-mediated pGSN expression in chemoresistant OVCA cells in an autocrine manner as well as confers cisplatin resistance in otherwise chemosensitive OVCA cells. These findings support our hypothesis that exosomal pGSN promotes OVCA cell survival through both autocrine and paracrine mechanisms that transform chemosensitive cells to resistant counterparts. Specifically, pGSN transported via exosomes is a determinant of chemoresistance in OVCA.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




Similar articles
-
The regulation of plasma gelsolin by DNA methylation in ovarian cancer chemo-resistance.J Ovarian Res. 2024 Jan 12;17(1):15. doi: 10.1186/s13048-023-01332-w. J Ovarian Res. 2024. PMID: 38216951 Free PMC article.
-
Exosomal Plasma Gelsolin Is an Immunosuppressive Mediator in the Ovarian Tumor Microenvironment and a Determinant of Chemoresistance.Cells. 2022 Oct 20;11(20):3305. doi: 10.3390/cells11203305. Cells. 2022. PMID: 36291171 Free PMC article. Review.
-
Plasma Gelsolin Inhibits CD8+ T-cell Function and Regulates Glutathione Production to Confer Chemoresistance in Ovarian Cancer.Cancer Res. 2020 Sep 15;80(18):3959-3971. doi: 10.1158/0008-5472.CAN-20-0788. Epub 2020 Jul 8. Cancer Res. 2020. PMID: 32641415
-
Plasma Gelsolin Confers Chemoresistance in Ovarian Cancer by Resetting the Relative Abundance and Function of Macrophage Subtypes.Cancers (Basel). 2022 Feb 18;14(4):1039. doi: 10.3390/cancers14041039. Cancers (Basel). 2022. PMID: 35205790 Free PMC article.
-
Exosomes: Emerging biomarkers and targets for ovarian cancer.Cancer Lett. 2015 Oct 10;367(1):26-33. doi: 10.1016/j.canlet.2015.07.014. Epub 2015 Jul 17. Cancer Lett. 2015. PMID: 26189430 Review.
Cited by
-
Gelsolin regulates intestinal stem cell regeneration and Th17 cellular function.Cell Commun Signal. 2024 Oct 29;22(1):524. doi: 10.1186/s12964-024-01902-5. Cell Commun Signal. 2024. PMID: 39472865 Free PMC article.
-
Circulating Plasma Gelsolin: A Predictor of Favorable Clinical Outcomes in Head and Neck Cancer and Sensitive Biomarker for Early Disease Diagnosis Combined with Soluble Fas Ligand.Cancers (Basel). 2020 Jun 13;12(6):1569. doi: 10.3390/cancers12061569. Cancers (Basel). 2020. PMID: 32545773 Free PMC article.
-
Extracellular Vesicles in Cancer Drug Resistance: Roles, Mechanisms, and Implications.Adv Sci (Weinh). 2022 Dec;9(34):e2201609. doi: 10.1002/advs.202201609. Epub 2022 Oct 17. Adv Sci (Weinh). 2022. PMID: 36253096 Free PMC article. Review.
-
The Role of Exosomes in Human Carcinogenesis and Cancer Therapy-Recent Findings from Molecular and Clinical Research.Cells. 2023 Jan 18;12(3):356. doi: 10.3390/cells12030356. Cells. 2023. PMID: 36766698 Free PMC article. Review.
-
SORT1/LAMP2-mediated extracellular vesicle secretion and cell adhesion are linked to lenalidomide resistance in multiple myeloma.Blood Adv. 2022 Apr 26;6(8):2480-2495. doi: 10.1182/bloodadvances.2021005772. Blood Adv. 2022. PMID: 34979567 Free PMC article.
References
-
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. - PubMed
-
- Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. - PubMed
-
- Yang X, Fraser M, Moll UM, Basak A, Tsang BK. Akt-mediated cisplatin resistance in ovarian cancer: modulation of p53 action on caspase-dependent mitochondrial death pathway. Cancer Res. 2006;66:3126–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

