Targeting cyclooxygenase by indomethacin decelerates progression of acute lymphoblastic leukemia in a xenograft model
- PMID: 31698450
- PMCID: PMC6855122
- DOI: 10.1182/bloodadvances.2019000473
Targeting cyclooxygenase by indomethacin decelerates progression of acute lymphoblastic leukemia in a xenograft model
Abstract
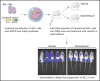
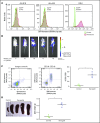
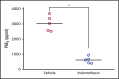
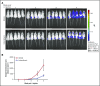
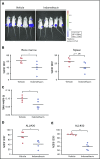
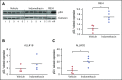
Acute lymphoblastic leukemia (ALL) develops in the bone marrow in the vicinity of stromal cells known to promote tumor development and treatment resistance. We previously showed that the cyclooxygenase (COX) inhibitor indomethacin prevents the ability of stromal cells to diminish p53-mediated killing of cocultured ALL cells in vitro, possibly by blocking the production of prostaglandin E2 (PGE2). Here, we propose that PGE2 released by bone marrow stromal cells might be a target for improved treatment of pediatric ALL. We used a xenograft model of human primary ALL cells in nonobese diabetic-scid IL2rγnull mice to show that indomethacin delivered in the drinking water delayed the progression of ALL in vivo. The progression was monitored by noninvasive in vivo imaging of the engrafted leukemic cells, as well as by analyses of CD19+CD10+ leukemic blasts present in spleen or bone marrow at the termination of the experiments. The indomethacin treatment increased the level of p53 in the leukemic cells, implying that COX inhibition might reduce progression of ALL by attenuating protective paracrine PGE2 signaling from bone marrow stroma to leukemic cells.
© 2019 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures
Similar articles
-
Suppression of bone marrow stromal cell function by benzene and hydroquinone is ameliorated by indomethacin.Toxicol Appl Pharmacol. 1987 Jul;89(3):378-90. doi: 10.1016/0041-008x(87)90157-8. Toxicol Appl Pharmacol. 1987. PMID: 3111015
-
Involvement of cyclooxygenase-derived prostaglandin E2 and nitric oxide in the protection of rat pancreas afforded by low dose of lipopolysaccharide.J Physiol Pharmacol. 2001 Mar;52(1):107-26. J Physiol Pharmacol. 2001. PMID: 11321505
-
Direct evidence for a role of cyclooxygenase 2-derived prostaglandin E2 in human head and neck xenograft tumors.Cancer Res. 2002 Nov 15;62(22):6706-11. Cancer Res. 2002. PMID: 12438270
-
Cyclooxygenase inhibitors retard murine mammary tumor progression by reducing tumor cell migration, invasiveness and angiogenesis.Int J Cancer. 2001 Aug 15;93(4):497-506. doi: 10.1002/ijc.1376. Int J Cancer. 2001. PMID: 11477553
-
The effect of selective cyclooxygenase inhibitors on intestinal epithelial cell mitogenesis.J Surg Res. 1999 Jan;81(1):101-7. doi: 10.1006/jsre.1998.5511. J Surg Res. 1999. PMID: 9889067
Cited by
-
Aspirin Induced Glioma Apoptosis through Noxa Upregulation.Int J Mol Sci. 2020 Jun 13;21(12):4219. doi: 10.3390/ijms21124219. Int J Mol Sci. 2020. PMID: 32545774 Free PMC article.
-
The Role of Prostaglandins in Different Types of Cancer.Cells. 2021 Jun 13;10(6):1487. doi: 10.3390/cells10061487. Cells. 2021. PMID: 34199169 Free PMC article. Review.
-
Targeting integrin αvβ3 with indomethacin inhibits patient-derived xenograft tumour growth and recurrence in oesophageal squamous cell carcinoma.Clin Transl Med. 2021 Oct;11(10):e548. doi: 10.1002/ctm2.548. Clin Transl Med. 2021. PMID: 34709754 Free PMC article.
References
-
- Curtin SC, Minino AM, Anderson RN. Declines in cancer death rates among children and adolescents in the United States, 1999-2014. NCHS Data Brief. 2016;(257):1-8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

