The Porcine Deltacoronavirus Replication Organelle Comprises Double-Membrane Vesicles and Zippered Endoplasmic Reticulum with Double-Membrane Spherules
- PMID: 31694296
- PMCID: PMC6893519
- DOI: 10.3390/v11111030
The Porcine Deltacoronavirus Replication Organelle Comprises Double-Membrane Vesicles and Zippered Endoplasmic Reticulum with Double-Membrane Spherules
Abstract
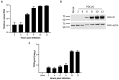
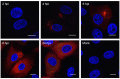
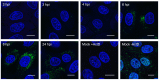
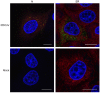
Porcine deltacoronavirus (PDCoV) was first identified in Hong Kong in 2012 from samples taken from pigs in 2009. PDCoV was subsequently identified in the USA in 2014 in pigs with a history of severe diarrhea. The virus has now been detected in pigs in several countries around the world. Following the development of tissue culture adapted strains of PDCoV, it is now possible to address questions regarding virus-host cell interactions for this genera of coronavirus. Here, we presented a detailed study of PDCoV-induced replication organelles. All positive-strand RNA viruses induce the rearrangement of cellular membranes during virus replication to support viral RNA synthesis, forming the replication organelle. Replication organelles for the Alpha-, Beta-, and Gammacoronavirus genera have been characterized. All coronavirus genera induced the formation of double-membrane vesicles (DMVs). In addition, Alpha- and Betacoronaviruses induce the formation of convoluted membranes, while Gammacoronaviruses induce the formation of zippered endoplasmic reticulum (ER) with tethered double-membrane spherules. However, the structures induced by Deltacoronaviruses, particularly the presence of convoluted membranes or double-membrane spherules, are unknown. Initially, the dynamics of PDCoV strain OH-FD22 replication were assessed with the onset of viral RNA synthesis, protein synthesis, and progeny particle release determined. Subsequently, virus-induced membrane rearrangements were identified in infected cells by electron microscopy. As has been observed for all other coronaviruses studied to date, PDCoV replication was found to induce the formation of double-membrane vesicles. Significantly, however, PDCoV replication was also found to induce the formation of regions of zippered endoplasmic reticulum, small associated tethered vesicles, and double-membrane spherules. These structures strongly resemble the replication organelle induced by avian Gammacoronavirus infectious bronchitis virus.
Keywords: DMV; coronavirus; double-membrane spherule; double-membrane vesicle; porcine deltacoronavirus; replication organelle; spherule; zippered ER.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
Infectious bronchitis virus generates spherules from zippered endoplasmic reticulum membranes.mBio. 2013 Oct 22;4(5):e00801-13. doi: 10.1128/mBio.00801-13. mBio. 2013. PMID: 24149513 Free PMC article.
-
Expression and Cleavage of Middle East Respiratory Syndrome Coronavirus nsp3-4 Polyprotein Induce the Formation of Double-Membrane Vesicles That Mimic Those Associated with Coronaviral RNA Replication.mBio. 2017 Nov 21;8(6):e01658-17. doi: 10.1128/mBio.01658-17. mBio. 2017. PMID: 29162711 Free PMC article.
-
Extensive coronavirus-induced membrane rearrangements are not a determinant of pathogenicity.Sci Rep. 2016 Jun 3;6:27126. doi: 10.1038/srep27126. Sci Rep. 2016. PMID: 27255716 Free PMC article.
-
Biogenesis and architecture of arterivirus replication organelles.Virus Res. 2016 Jul 15;220:70-90. doi: 10.1016/j.virusres.2016.04.001. Epub 2016 Apr 9. Virus Res. 2016. PMID: 27071852 Free PMC article. Review.
-
The double-membrane vesicle (DMV): a virus-induced organelle dedicated to the replication of SARS-CoV-2 and other positive-sense single-stranded RNA viruses.Cell Mol Life Sci. 2022 Jul 16;79(8):425. doi: 10.1007/s00018-022-04469-x. Cell Mol Life Sci. 2022. PMID: 35841484 Free PMC article. Review.
Cited by
-
Multiscale Electron Microscopy for the Study of Viral Replication Organelles.Viruses. 2021 Jan 28;13(2):197. doi: 10.3390/v13020197. Viruses. 2021. PMID: 33525547 Free PMC article. Review.
-
Double-Membrane Vesicles as Platforms for Viral Replication.Trends Microbiol. 2020 Dec;28(12):1022-1033. doi: 10.1016/j.tim.2020.05.009. Epub 2020 Jun 11. Trends Microbiol. 2020. PMID: 32536523 Free PMC article. Review.
-
A novel optimized pre-embedding antibody-labelling correlative light electron microscopy technique.Access Microbiol. 2024 Feb 20;6(2):000750.v3. doi: 10.1099/acmi.0.000750.v3. eCollection 2024. Access Microbiol. 2024. PMID: 38482358 Free PMC article.
-
Coronavirus infection induces progressive restructuring of the endoplasmic reticulum involving the formation and degradation of double membrane vesicles.Virology. 2021 Apr;556:9-22. doi: 10.1016/j.virol.2020.12.007. Epub 2020 Dec 24. Virology. 2021. PMID: 33524849 Free PMC article.
-
Hunting coronavirus by transmission electron microscopy - a guide to SARS-CoV-2-associated ultrastructural pathology in COVID-19 tissues.Histopathology. 2021 Feb;78(3):358-370. doi: 10.1111/his.14264. Epub 2020 Dec 1. Histopathology. 2021. PMID: 32981112 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- BB/N002350/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/I/00007039/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/I/00007037/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/I/00007034/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/I/00007038/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Research Materials

