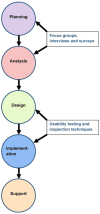
Key methodological considerations for usability testing of electronic patient-reported outcome (ePRO) systems
- PMID: 31691202
- PMCID: PMC6994506
- DOI: 10.1007/s11136-019-02329-z
Key methodological considerations for usability testing of electronic patient-reported outcome (ePRO) systems
Abstract
Introduction: Recent advances in information technology and improved access to the internet have led to a rapid increase in the adoption and ownership of electronic devices such as touch screen smartphones and tablet computers. This has also led to a renewed interest in the field of digital health also referred to as telehealth or electronic health (eHealth). There is now a drive to collect these PROs electronically using ePRO systems.
Method: However, the user interfaces of ePRO systems need to be adequately assessed to ensure they are not only fit for purpose but also acceptable to patients who are the end users. Usability testing is a technique that involves the testing of systems, products or websites with participants drawn from the target population. Usability testing can assist ePRO developers in the evaluation of ePRO user interface. The complexity of ePRO systems; stage of development; metrics to measure; and the use of scenarios, moderators and appropriate sample sizes are key methodological issues to consider when planning usability tests.
Conclusion: The findings from usability testing may facilitate the improvement of ePRO systems making them more usable and acceptable to end users. This may in turn improve the adoption of ePRO systems post-implementation. This article highlights the key methodological issues to consider and address when planning usability testing of ePRO systems.
Keywords: Digital health; Electronic patient-reported outcomes; Electronic systems; PROs; Telehealth; Usability testing; eHealth; ePRO systems; ePROM; ePROs.
Conflict of interest statement
The author declares that there is no conflict of interest with respect to the research, authorship and/or publication of this article.
Figures
Similar articles
-
Development and usability testing of an electronic patient-reported outcome (ePRO) solution for patients with inflammatory diseases in an Advanced Therapy Medicinal Product (ATMP) basket trial.J Patient Rep Outcomes. 2023 Oct 9;7(1):98. doi: 10.1186/s41687-023-00634-3. J Patient Rep Outcomes. 2023. PMID: 37812323 Free PMC article.
-
Usability Testing of an Electronic Patient-Reported Outcome System for Survivors of Critical Illness.Am J Crit Care. 2016 Jul;25(4):340-9. doi: 10.4037/ajcc2016952. Am J Crit Care. 2016. PMID: 27369033
-
The Electronic Patient Reported Outcome Tool: Testing Usability and Feasibility of a Mobile App and Portal to Support Care for Patients With Complex Chronic Disease and Disability in Primary Care Settings.JMIR Mhealth Uhealth. 2016 Jun 2;4(2):e58. doi: 10.2196/mhealth.5331. JMIR Mhealth Uhealth. 2016. PMID: 27256035 Free PMC article.
-
Global use of electronic patient-reported outcome systems in nephrology: a mixed methods study.BMJ Open. 2023 Jul 12;13(7):e070927. doi: 10.1136/bmjopen-2022-070927. BMJ Open. 2023. PMID: 37438075 Free PMC article. Review.
-
A Narrative Review on the Collection and Use of Electronic Patient-Reported Outcomes in Cancer Survivorship Care with Emphasis on Symptom Monitoring.Curr Oncol. 2022 Jun 17;29(6):4370-4385. doi: 10.3390/curroncol29060349. Curr Oncol. 2022. PMID: 35735458 Free PMC article. Review.
Cited by
-
Interprofessional Collaboration and Diabetes Management in Primary Care: A Systematic Review and Meta-Analysis of Patient-Reported Outcomes.J Pers Med. 2022 Apr 15;12(4):643. doi: 10.3390/jpm12040643. J Pers Med. 2022. PMID: 35455759 Free PMC article. Review.
-
Graphical user interface design to improve understanding of the patient-reported outcome symptom response.PLoS One. 2023 Jan 24;18(1):e0278465. doi: 10.1371/journal.pone.0278465. eCollection 2023. PLoS One. 2023. PMID: 36693053 Free PMC article.
-
Data Quality of Longitudinally Collected Patient-Reported Outcomes After Thoracic Surgery: Comparison of Paper- and Web-Based Assessments.J Med Internet Res. 2021 Nov 9;23(11):e28915. doi: 10.2196/28915. J Med Internet Res. 2021. PMID: 34751657 Free PMC article. Clinical Trial.
-
Development of a telemedicine virtual clinic system for remote, rural, and underserved areas using user-centered design methods.Digit Health. 2024 May 26;10:20552076241256752. doi: 10.1177/20552076241256752. eCollection 2024 Jan-Dec. Digit Health. 2024. PMID: 38812852 Free PMC article.
-
A narrative review of current evidence supporting the implementation of electronic patient-reported outcome measures in the management of chronic diseases.Ther Adv Chronic Dis. 2021 May 24;12:20406223211015958. doi: 10.1177/20406223211015958. eCollection 2021. Ther Adv Chronic Dis. 2021. PMID: 34104376 Free PMC article.
References
-
- Perrin A. 10 facts about smartphones as the iPhone turns 10: Pew Research Center; 2017. Retrieved from October 2018 http://www.pewresearch.org/fact-tank/2017/06/28/10-facts-about-smartphones/.
-
- Hong YA, Cho J. Has the digital health divide widened? Trends of health-related internet use among older adults from 2003 to 2011. The Journals of Gerontology: Series B. 2017;72(5):856–863. - PubMed
-
- FDA. Guidance for industry. Patient-reported outcome measures: Use in medicinal product development to support labeling claims. Silver Spring, MD: US Department of Health and Human Services Food and Drug Administration; 2009. - PubMed
-
- Basch E, Geoghegan C, Coons SJ, Gnanasakthy A, Slagle AF, Papadopoulos EJ, et al. Patient-reported outcomes in cancer drug development and US regulatory review: Perspectives from industry, the food and drug administration, and the patient. JAMA Oncology. 2015;1(3):375–379. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous