Bovine Herpesvirus 1 Productive Infection Led to Inactivation of Nrf2 Signaling through Diverse Approaches
- PMID: 31687081
- PMCID: PMC6800938
- DOI: 10.1155/2019/4957878
Bovine Herpesvirus 1 Productive Infection Led to Inactivation of Nrf2 Signaling through Diverse Approaches
Abstract
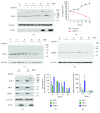
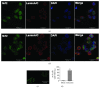
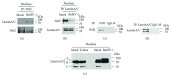
Bovine herpesvirus type 1 (BoHV-1) is a significant cofactor for bovine respiratory disease complex (BRDC), the most important inflammatory disease in cattle. BoHV-1 infection in cell cultures induces overproduction of pathogenic reactive oxygen species (ROS) and the depletion of nuclear factor erythroid 2 p45-related factor 2 (Nrf2), a master transcriptional factor regulating a panel of antioxidant and cellular defense genes in response to oxidative stress. In this study, we reported that the virus productive infection in MDBK cells at the later stage significantly decreased the expression levels of heme oxygenase-1 (HO-1) and NAD(P)H quinone oxidoreductase-1 (NQO1) proteins, the canonical downstream targets regulated by Nrf2, inhibited Nrf2 acetylation, reduced the accumulation of Nrf2 proteins in the nucleus, and relocalized nuclear Nrf2 proteins to form dot-like staining patterns in confocal microscope assay. The differential expression of Kelch-like ECH associated protein 1 (KEAP1) and DJ-1 proteins as well as the decreased association between KEAP1 and DJ-1 promoted Nrf2 degradation through the ubiquitin proteasome pathway. These data indicated that the BoHV-1 infection may significantly suppress the Nrf2 signaling pathway. Moreover, we found that there was an association between Nrf2 and LaminA/C, H3K9ac, and H3K18ac, and the binding ratios were altered following the virus infection. Taken together, for the first time, we provided evidence showing that BoHV-1 infection inhibited the Nrf2 signaling pathway by complicated mechanisms including promoting Nrf2 degradation, relocalization of nuclear Nrf2, and inhibition of Nrf2 acetylation.
Copyright © 2019 Xiaotian Fu et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





Similar articles
-
The Involvement of Histone H3 Acetylation in Bovine Herpesvirus 1 Replication in MDBK Cells.Viruses. 2018 Sep 27;10(10):525. doi: 10.3390/v10100525. Viruses. 2018. PMID: 30261679 Free PMC article.
-
Tetrachlorobenzoquinone activates Nrf2 signaling by Keap1 cross-linking and ubiquitin translocation but not Keap1-Cullin3 complex dissociation.Chem Res Toxicol. 2015 Apr 20;28(4):765-74. doi: 10.1021/tx500513v. Epub 2015 Mar 11. Chem Res Toxicol. 2015. PMID: 25742418
-
NFAT5 Restricts Bovine Herpesvirus 1 Productive Infection in MDBK Cell Cultures.Microbiol Spectr. 2023 Aug 17;11(4):e0011723. doi: 10.1128/spectrum.00117-23. Epub 2023 May 25. Microbiol Spectr. 2023. PMID: 37227295 Free PMC article.
-
The Keap1-Nrf2-antioxidant response element pathway: a review of its regulation by melatonin and the proteasome.Mol Cell Endocrinol. 2015 Feb 5;401:213-20. doi: 10.1016/j.mce.2014.12.013. Epub 2014 Dec 17. Mol Cell Endocrinol. 2015. PMID: 25528518 Review.
-
Canonical and non-canonical mechanisms of Nrf2 activation.Pharmacol Res. 2018 Aug;134:92-99. doi: 10.1016/j.phrs.2018.06.013. Epub 2018 Jun 18. Pharmacol Res. 2018. PMID: 29913224 Review.
Cited by
-
Upregulation of nuclear factor E2-related factor 2 (Nrf2) represses the replication of herpes simplex virus type 1.Virol J. 2022 Jan 31;19(1):23. doi: 10.1186/s12985-021-01733-7. Virol J. 2022. PMID: 35101046 Free PMC article.
-
Arsenite Inhibits Tissue-Type Plasminogen Activator Synthesis through NRF2 Activation in Cultured Human Vascular Endothelial EA.hy926 Cells.Int J Mol Sci. 2021 Jan 13;22(2):739. doi: 10.3390/ijms22020739. Int J Mol Sci. 2021. PMID: 33451022 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

