Emerging approaches to target mitochondrial apoptosis in cancer cells
- PMID: 31681471
- PMCID: PMC6816453
- DOI: 10.12688/f1000research.18872.1
Emerging approaches to target mitochondrial apoptosis in cancer cells
Abstract
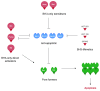
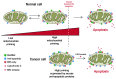
Apoptosis is a highly conserved programme for removing damaged and unwanted cells. Apoptosis in most cells is coordinated on mitochondria by the Bcl-2 family of proteins. The balance between pro- and anti-apoptotic Bcl-2 family proteins sets a threshold for mitochondrial apoptosis, a balance that is altered during cancer progression. Consequently, avoidance of cell death is an established cancer hallmark. Although there is a general perception that tumour cells are more resistant to apoptosis than their normal counterparts, the realities of cell death regulation in cancer are more nuanced. In this review we discuss how a profound understanding of this control has led to new therapeutic approaches, including the new class of BH3-mimetics, which directly target apoptosis as a vulnerability in cancer. We discuss recent findings that highlight the current limitations in our understanding of apoptosis and how these novel therapeutics work.
Keywords: Apoptosis; BH3-mimetics; cancer.
Copyright: © 2019 Gilmore A and King L.
Conflict of interest statement
No competing interests were disclosed.No competing interests were disclosed.No competing interests were disclosed.No competing interests were disclosed.
Figures
Similar articles
-
Targeting BCL-2-like Proteins to Kill Cancer Cells.Trends Cancer. 2016 Aug;2(8):443-460. doi: 10.1016/j.trecan.2016.07.001. Epub 2016 Jul 30. Trends Cancer. 2016. PMID: 28741496 Review.
-
Targeting BCL-2 to enhance vulnerability to therapy in estrogen receptor-positive breast cancer.Oncogene. 2016 Apr 14;35(15):1877-87. doi: 10.1038/onc.2015.287. Epub 2015 Aug 10. Oncogene. 2016. PMID: 26257067 Review.
-
The BCL-2 arbiters of apoptosis and their growing role as cancer targets.Cell Death Differ. 2018 Jan;25(1):27-36. doi: 10.1038/cdd.2017.161. Epub 2017 Nov 3. Cell Death Differ. 2018. PMID: 29099483 Free PMC article. Review.
-
A BH3 Mimetic for Killing Cancer Cells.Cell. 2016 Jun 16;165(7):1560. doi: 10.1016/j.cell.2016.05.080. Cell. 2016. PMID: 27315468
-
Side-by-side comparison of BH3-mimetics identifies MCL-1 as a key therapeutic target in AML.Cell Death Dis. 2019 Dec 4;10(12):917. doi: 10.1038/s41419-019-2156-2. Cell Death Dis. 2019. PMID: 31801941 Free PMC article.
Cited by
-
The Association of TP53, BCL2, BAX and NOXA SNPs and Laryngeal Squamous Cell Carcinoma Development.Int J Mol Sci. 2024 Nov 4;25(21):11849. doi: 10.3390/ijms252111849. Int J Mol Sci. 2024. PMID: 39519400 Free PMC article.
-
Role of Mitochondria and Lysosomes in the Selective Cytotoxicity of Cold Atmospheric Plasma on Retinoblastoma Cells.Iran J Pharm Res. 2020 Fall;19(4):203-215. doi: 10.22037/ijpr.2020.114165.14703. Iran J Pharm Res. 2020. PMID: 33841536 Free PMC article.
-
Mitochondrial Metabolism: A New Dimension of Personalized Oncology.Cancers (Basel). 2023 Aug 11;15(16):4058. doi: 10.3390/cancers15164058. Cancers (Basel). 2023. PMID: 37627086 Free PMC article. Review.
-
Gene Therapy with MiRNA-Mediated Targeting of Mcl-1 Promotes the Sensitivity of Non-Small Cell Lung Cancer Cells to Treatment with ABT-737.Asian Pac J Cancer Prev. 2020 Mar 1;21(3):675-681. doi: 10.31557/APJCP.2020.21.3.675. Asian Pac J Cancer Prev. 2020. PMID: 32212793 Free PMC article.
-
Blockage of glycolysis by targeting PFKFB3 suppresses the development of infantile hemangioma.J Transl Med. 2023 Feb 6;21(1):85. doi: 10.1186/s12967-023-03932-y. J Transl Med. 2023. PMID: 36740704 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

