Compromised Bone Healing in Aged Rats Is Associated With Impaired M2 Macrophage Function
- PMID: 31681320
- PMCID: PMC6813416
- DOI: 10.3389/fimmu.2019.02443
Compromised Bone Healing in Aged Rats Is Associated With Impaired M2 Macrophage Function
Abstract
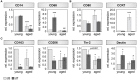
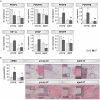
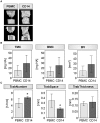
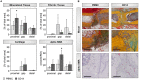
Fracture repair is initiated by a multitude of immune cells and induction of an inflammatory cascade. Alterations in the early healing response due to an aged adaptive immune system leads to impaired bone repair, delayed healing or even formation of non-union. However, immuno-senescence is not limited to the adaptive immunity, but is also described for macrophages, main effector cells from the innate immune system. Beside regulation of pro- and anti-inflammatory signaling, macrophages contribute to angiogenesis and granulation tissue maturation. Thus, it seems likely that an altered macrophage function due to aging may affect bone repair at various stages and contribute to age related deficiencies in bone regeneration. To prove this hypothesis, we analyzed the expression of macrophage markers and angiogenic factors in the early bone hematoma derived from young and aged osteotomized Spraque Dawley rats. We detected an overall reduced expression of the monocyte/pan-macrophage markers CD14 and CD68 in aged rats. Furthermore, the analysis revealed an impaired expression of anti-inflammatory M2 macrophage markers in hematoma from aged animals that was connected to a diminished revascularization of the bone callus. To verify that the age related disturbed bone regeneration was due to a compromised macrophage function, CD14+ macrophage precursors were transplanted locally into the osteotomy gap of aged rats. Transplantation rescued bone regeneration partially after 6 weeks, demonstrated by a significantly induced deposition of new bone tissue, reduced fibrosis and significantly improved callus vascularization.
Keywords: CD14+ cells; aging; angiogenesis; bone regeneration; compromised healing; macrophage; monocyte.
Copyright © 2019 Löffler, Sass, Filter, Rose, Ellinghaus, Duda and Dienelt.
Figures
Similar articles
-
Macrophage polarisation: an immunohistochemical approach for identifying M1 and M2 macrophages.PLoS One. 2013 Nov 15;8(11):e80908. doi: 10.1371/journal.pone.0080908. eCollection 2013. PLoS One. 2013. PMID: 24260507 Free PMC article.
-
A Biomimetic Hierarchical Nanointerface Orchestrates Macrophage Polarization and Mesenchymal Stem Cell Recruitment To Promote Endogenous Bone Regeneration.ACS Nano. 2019 Jun 25;13(6):6581-6595. doi: 10.1021/acsnano.9b00489. Epub 2019 May 31. ACS Nano. 2019. PMID: 31125522
-
Cardiac CD68+ and stabilin-1+ macrophages in wound healing following myocardial infarction: From experiment to clinic.Immunobiology. 2018 Apr-May;223(4-5):413-421. doi: 10.1016/j.imbio.2017.11.006. Epub 2017 Nov 22. Immunobiology. 2018. PMID: 29179985
-
Osteomacs and Bone Regeneration.Curr Osteoporos Rep. 2017 Aug;15(4):385-395. doi: 10.1007/s11914-017-0384-x. Curr Osteoporos Rep. 2017. PMID: 28647885 Review.
-
The multifaceted roles of macrophages in bone regeneration: A story of polarization, activation and time.Acta Biomater. 2021 Oct 1;133:46-57. doi: 10.1016/j.actbio.2021.04.052. Epub 2021 May 8. Acta Biomater. 2021. PMID: 33974949 Review.
Cited by
-
Monocyte alteration in elderly hip fracture healing: monocyte promising role in bone regeneration.Immun Ageing. 2024 Feb 3;21(1):12. doi: 10.1186/s12979-024-00413-8. Immun Ageing. 2024. PMID: 38308312 Free PMC article. Review.
-
Bone Healing Gone Wrong: Pathological Fracture Healing and Non-Unions-Overview of Basic and Clinical Aspects and Systematic Review of Risk Factors.Bioengineering (Basel). 2023 Jan 9;10(1):85. doi: 10.3390/bioengineering10010085. Bioengineering (Basel). 2023. PMID: 36671657 Free PMC article. Review.
-
Modulating the systemic and local adaptive immune response after fracture improves bone regeneration during aging.Bone. 2022 Apr;157:116324. doi: 10.1016/j.bone.2021.116324. Epub 2022 Jan 6. Bone. 2022. PMID: 34998981 Free PMC article.
-
E-cigarette Aerosol Mixtures Inhibit Biomaterial-Induced Osseointegrative Cell Phenotypes.Materialia (Oxf). 2021 Dec;20:101241. doi: 10.1016/j.mtla.2021.101241. Epub 2021 Oct 8. Materialia (Oxf). 2021. PMID: 34778733 Free PMC article.
-
Macrophages-bone marrow mesenchymal stem cells crosstalk in bone healing.Front Cell Dev Biol. 2023 Jun 23;11:1193765. doi: 10.3389/fcell.2023.1193765. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37427382 Free PMC article. Review.
References
-
- Tarantino U, Cerocchi I, Scialdoni A, Saturnino L, Feola M, Celi M, et al. . Bone healing and osteoporosis. Aging Clin Exp Res. (2011) 23(Suppl 2):62–41. Available online at: https://www.researchgate.net/publication/51693253_Bone_healing_and_osteo... - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

