Is There a Role for GPCR Agonist Radiotracers in PET Neuroimaging?
- PMID: 31680859
- PMCID: PMC6813225
- DOI: 10.3389/fnmol.2019.00255
Is There a Role for GPCR Agonist Radiotracers in PET Neuroimaging?
Abstract
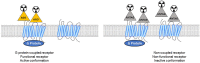
Positron emission tomography (PET) is a molecular imaging modality that enables in vivo exploration of metabolic processes and especially the pharmacology of neuroreceptors. G protein-coupled receptors (GPCRs) play an important role in numerous pathophysiologic disorders of the central nervous system. Thus, they are targets of choice in PET imaging to bring proof concept of change in density in pathological conditions or in pharmacological challenge. At present, most radiotracers are antagonist ligands. In vitro data suggest that properties differ between GPCR agonists and antagonists: antagonists bind to receptors with a single affinity, whereas agonists are characterized by two different affinities: high affinity for receptors that undergo functional coupling to G-proteins, and low affinity for those that are not coupled. In this context, agonist radiotracers may be useful tools to give functional images of GPCRs in the brain, with high sensitivity to neurotransmitter release. Here, we review all existing PET radiotracers used from animals to humans and their role for understanding the ligand-receptor paradigm of GPCR in comparison with corresponding antagonist radiotracers.
Keywords: Positron emission tomography (PET); agonist; neuroimaging; radiopharmaceutical; receptor.
Copyright © 2019 Colom, Vidal and Zimmer.
Figures

Similar articles
-
18F-F13640 preclinical evaluation in rodent, cat and primate as a 5-HT1A receptor agonist for PET neuroimaging.Brain Struct Funct. 2018 Jul;223(6):2973-2988. doi: 10.1007/s00429-018-1672-7. Epub 2018 May 5. Brain Struct Funct. 2018. PMID: 29730825
-
Agonist and antagonist bind differently to 5-HT1A receptors during Alzheimer's disease: A post-mortem study with PET radiopharmaceuticals.Neuropharmacology. 2016 Oct;109:88-95. doi: 10.1016/j.neuropharm.2016.05.009. Epub 2016 May 13. Neuropharmacology. 2016. PMID: 27183968
-
Synthesis, in vitro and in vivo evaluation of 11C-O-methylated arylpiperazines as potential serotonin 1A (5-HT1A) receptor antagonist radiotracers.EJNMMI Radiopharm Chem. 2020 May 19;5(1):13. doi: 10.1186/s41181-020-00096-8. EJNMMI Radiopharm Chem. 2020. PMID: 32430632 Free PMC article.
-
Hunting for the high-affinity state of G-protein-coupled receptors with agonist tracers: Theoretical and practical considerations for positron emission tomography imaging.Med Res Rev. 2019 May;39(3):1014-1052. doi: 10.1002/med.21552. Epub 2018 Nov 18. Med Res Rev. 2019. PMID: 30450619 Free PMC article. Review.
-
Current status of positron emission tomography radiotracers for serotonin receptors in humans.J Labelled Comp Radiopharm. 2013 Mar-Apr;56(3-4):105-13. doi: 10.1002/jlcr.3001. J Labelled Comp Radiopharm. 2013. PMID: 24285316 Review.
Cited by
-
Opposite alterations of 5-HT2A receptor brain density in subjects with schizophrenia: relevance of radiotracers pharmacological profile.Transl Psychiatry. 2021 May 20;11(1):302. doi: 10.1038/s41398-021-01430-7. Transl Psychiatry. 2021. PMID: 34016955 Free PMC article.
-
Design and validation of recombinant protein standards for quantitative Western blot analysis of cannabinoid CB1 receptor density in cell membranes: an alternative to radioligand binding methods.Microb Cell Fact. 2022 Sep 15;21(1):192. doi: 10.1186/s12934-022-01914-1. Microb Cell Fact. 2022. PMID: 36109736 Free PMC article.
-
Assessing the impact of different penalty factors of the Bayesian reconstruction algorithm Q.Clear on in vivo low count kinetic analysis of [11C]PHNO brain PET-MR studies.EJNMMI Res. 2022 Feb 20;12(1):11. doi: 10.1186/s13550-022-00883-1. EJNMMI Res. 2022. PMID: 35184229 Free PMC article.
-
Peering into the Brain's Estrogen Receptors: PET Tracers for Visualization of Nuclear and Extranuclear Estrogen Receptors in Brain Disorders.Biomolecules. 2023 Sep 18;13(9):1405. doi: 10.3390/biom13091405. Biomolecules. 2023. PMID: 37759805 Free PMC article.
-
PET imaging of neural activity, β-amyloid, and tau in normal brain aging.Eur J Nucl Med Mol Imaging. 2021 Nov;48(12):3859-3871. doi: 10.1007/s00259-021-05230-5. Epub 2021 Mar 5. Eur J Nucl Med Mol Imaging. 2021. PMID: 33674892 Review.
References
Publication types
LinkOut - more resources
Full Text Sources

