ASCs-Exosomes Recover Coupling Efficiency and Mitochondrial Membrane Potential in an in vitro Model of ALS
- PMID: 31680811
- PMCID: PMC6811497
- DOI: 10.3389/fnins.2019.01070
ASCs-Exosomes Recover Coupling Efficiency and Mitochondrial Membrane Potential in an in vitro Model of ALS
Abstract
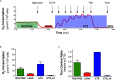
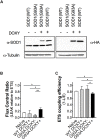
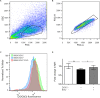
The amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disorder characterized by motoneurons death. Mutations in the superoxide dismutase 1 (SOD1) protein have been identified to be related to the disease. Beyond the different altered pathways, the mitochondrial dysfunction is one of the major features that leads to the selective death of motoneurons in ALS. The NSC-34 cell line, overexpressing human SOD1(G93A) mutant protein [NSC-34(G93A)], is considered an optimal in vitro model to study ALS. Here we investigated the energy metabolism in NSC-34(G93A) cells and in particular the effect of the mutated SOD1(G93A) protein on the mitochondrial respiratory capacity (complexes I-IV) by high resolution respirometry (HRR) and cytofluorimetry. We demonstrated that NSC-34(G93A) cells show a reduced mitochondrial oxidative capacity. In particular, we found significant impairment of the complex I-linked oxidative phosphorylation, reduced efficiency of the electron transfer system (ETS) associated with a higher rate of dissipative respiration, and a lower membrane potential. In order to rescue the effect of the mutated SOD1 gene on mitochondria impairment, we evaluated the efficacy of the exosomes, isolated from adipose-derived stem cells, administrated on the NSC-34(G93A) cells. These data show that ASCs-exosomes are able to restore complex I activity, coupling efficiency and mitochondrial membrane potential. Our results improve the knowledge about mitochondrial bioenergetic defects directly associated with the SOD1(G93A) mutation, and prove the efficacy of adipose-derived stem cells exosomes to rescue the function of mitochondria, indicating that these vesicles could represent a valuable approach to target mitochondrial dysfunction in ALS.
Keywords: ALS; NSC-34 cell line; complex I; coupling efficiency; exosomes; high resolution respirometry; membrane potential; mitochondria.
Copyright © 2019 Calabria, Scambi, Bonafede, Schiaffino, Peroni, Potrich, Capelli, Schena and Mariotti.
Figures



Similar articles
-
ALS-associated mutation SOD1G93A leads to abnormal mitochondrial dynamics in osteocytes.Bone. 2018 Jan;106:126-138. doi: 10.1016/j.bone.2017.10.010. Epub 2017 Oct 10. Bone. 2018. PMID: 29030231 Free PMC article.
-
Cell culture models to investigate the selective vulnerability of motoneuronal mitochondria to familial ALS-linked G93ASOD1.Eur J Neurosci. 2006 Jul;24(2):387-99. doi: 10.1111/j.1460-9568.2006.04922.x. Eur J Neurosci. 2006. PMID: 16903849
-
Exosome derived from murine adipose-derived stromal cells: Neuroprotective effect on in vitro model of amyotrophic lateral sclerosis.Exp Cell Res. 2016 Jan 1;340(1):150-8. doi: 10.1016/j.yexcr.2015.12.009. Epub 2015 Dec 18. Exp Cell Res. 2016. PMID: 26708289
-
Increased mitochondrial antioxidative activity or decreased oxygen free radical propagation prevent mutant SOD1-mediated motor neuron cell death and increase amyotrophic lateral sclerosis-like transgenic mouse survival.J Neurochem. 2002 Feb;80(3):488-500. doi: 10.1046/j.0022-3042.2001.00720.x. J Neurochem. 2002. PMID: 11905995
-
Mutant SOD1 mediated pathogenesis of Amyotrophic Lateral Sclerosis.Gene. 2016 Feb 15;577(2):109-18. doi: 10.1016/j.gene.2015.11.049. Epub 2015 Dec 2. Gene. 2016. PMID: 26657039 Review.
Cited by
-
Adipose-Derived Mesenchymal Stem Cells Combined With Extracellular Vesicles May Improve Amyotrophic Lateral Sclerosis.Front Aging Neurosci. 2022 May 18;14:830346. doi: 10.3389/fnagi.2022.830346. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35663577 Free PMC article. Review.
-
Released Exosomes Contribute to the Immune Modulation of Cord Blood-Derived Stem Cells.Front Immunol. 2020 Feb 25;11:165. doi: 10.3389/fimmu.2020.00165. eCollection 2020. Front Immunol. 2020. PMID: 32161585 Free PMC article.
-
Extracellular Vesicles, Cell-Penetrating Peptides and miRNAs as Future Novel Therapeutic Interventions for Parkinson's and Alzheimer's Disease.Biomedicines. 2023 Feb 28;11(3):728. doi: 10.3390/biomedicines11030728. Biomedicines. 2023. PMID: 36979707 Free PMC article. Review.
-
Advances in treatment of neurodegenerative diseases: Perspectives for combination of stem cells with neurotrophic factors.World J Stem Cells. 2020 May 26;12(5):323-338. doi: 10.4252/wjsc.v12.i5.323. World J Stem Cells. 2020. PMID: 32547681 Free PMC article. Review.
-
Extracellular Vesicles: The Next Generation of Biomarkers and Treatment for Central Nervous System Diseases.Int J Mol Sci. 2024 Jul 5;25(13):7371. doi: 10.3390/ijms25137371. Int J Mol Sci. 2024. PMID: 39000479 Free PMC article. Review.
References
-
- Allen S. P., Rajan S., Duffy L., Mortiboys H., Higginbottom A., Grierson A. J., et al. (2014). Superoxide dismutase 1 mutation in a cellular model of amyotrophic lateral sclerosis shifts energy generation from oxidative phosphorylation to glycolysis. Neurobiol. Aging 35 1499–1509. 10.1016/j.neurobiolaging.2013 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

