Hypermethylation of mismatch repair gene hMSH2 associates with platinum-resistant disease in epithelial ovarian cancer
- PMID: 31666131
- PMCID: PMC6822346
- DOI: 10.1186/s13148-019-0748-4
Hypermethylation of mismatch repair gene hMSH2 associates with platinum-resistant disease in epithelial ovarian cancer
Abstract
Purpose: One major reason of the high mortality of epithelial ovarian cancer (EOC) is due to platinum-based chemotherapy resistance. Aberrant DNA methylation may be a potential mechanism underlying the development of platinum resistance in EOC. The purpose of this study is to discover potential aberrant DNA methylation that contributes to drug resistance.
Methods: By initially screening of 16 platinum-sensitive/resistant samples from EOC patients with reduced representation bisulfite sequencing (RRBS), the upstream region of the hMSH2 gene was discovered hypermethylated in the platinum-resistant group. The effect of hMSH2 methylation on the cellular response to cisplatin was explored by demethylation and knockdown assays in ovarian cancer cell line A2780. Matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry was employed to examine the methylation levels of hMSH2 upstream region in additional 40 EOC patient samples. RT-qPCR and IHC assay was used to detect the hMSH2 mRNA and protein expression in extended 150 patients.
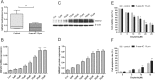
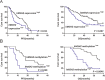
Results: RRBS assay discovered an upstream region from - 1193 to - 1125 of hMSH2 was significant hypermethylated in resistant EOC patients (P = 1.06 × 10-14). In vitro analysis demonstrated that global demethylation increased cisplatin sensitivity along with a higher expression of the hMSH2 mRNA and protein. Knockdown hMSH2 reduced the cell sensitivity to cisplatin. MALDI-TOF mass spectrometry assay validated the strong association of hypermethylation of hMSH2 upstream region with platinum resistance. Spearman's correlation analysis revealed a significantly negative connection between methylation level of hMSH2 upstream region and its expression. The Kaplan-Meier analyses showed the high methylation of hMSH2 promoter region, and its low expressions are associated with worse survival. In multivariable models, hMSH2 low expression was an independent factor predicting poor outcome (P = 0.03, HR = 1.91, 95%CI = 1.85-2.31).
Conclusion: The hypermethylation of hMSH2 upstream region is associated with platinum resistant in EOC, and low expression of hMSH2 may be an index for the poor prognosis.
Keywords: DNA methylation; Mismatch repair; Prognosis; RRBS; hMSH2.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
hMSH2 coordinated with the expression of E2F1 promotes platinum response in epithelial ovarian cancer.J Int Med Res. 2023 Mar;51(3):3000605231163780. doi: 10.1177/03000605231163780. J Int Med Res. 2023. PMID: 36994850 Free PMC article.
-
[Clinical and bioinformatics analysis of the relationship between LAMA3 DNA methylation expression and platinum resistance and prognosis in epithelial ovarian cancer].Zhonghua Fu Chan Ke Za Zhi. 2024 Jun 25;59(6):454-464. doi: 10.3760/cma.j.cn112141-20240201-00069. Zhonghua Fu Chan Ke Za Zhi. 2024. PMID: 38951081 Chinese.
-
Transcriptional epigenetic regulation of Fkbp1/Pax9 genes is associated with impaired sensitivity to platinum treatment in ovarian cancer.Clin Epigenetics. 2021 Aug 28;13(1):167. doi: 10.1186/s13148-021-01149-8. Clin Epigenetics. 2021. PMID: 34454589 Free PMC article.
-
Downregulation of RIF1 Enhances Sensitivity to Platinum-Based Chemotherapy in Epithelial Ovarian Cancer (EOC) by Regulating Nucleotide Excision Repair (NER) Pathway.Cell Physiol Biochem. 2018;46(5):1971-1984. doi: 10.1159/000489418. Epub 2018 Apr 26. Cell Physiol Biochem. 2018. PMID: 29719287
-
Role of Epigenetics for the Efficacy of Cisplatin.Int J Mol Sci. 2024 Jan 17;25(2):1130. doi: 10.3390/ijms25021130. Int J Mol Sci. 2024. PMID: 38256203 Free PMC article. Review.
Cited by
-
DNA Repair and Ovarian Carcinogenesis: Impact on Risk, Prognosis and Therapy Outcome.Cancers (Basel). 2020 Jun 28;12(7):1713. doi: 10.3390/cancers12071713. Cancers (Basel). 2020. PMID: 32605254 Free PMC article. Review.
-
DNA Methylation Biomarkers for Prediction of Response to Platinum-Based Chemotherapy: Where Do We Stand?Cancers (Basel). 2022 Jun 13;14(12):2918. doi: 10.3390/cancers14122918. Cancers (Basel). 2022. PMID: 35740584 Free PMC article. Review.
-
MiR-590-5p promotes cisplatin resistance via targeting hMSH2 in ovarian cancer.Mol Biol Rep. 2023 Aug;50(8):6819-6827. doi: 10.1007/s11033-023-08599-8. Epub 2023 Jul 1. Mol Biol Rep. 2023. PMID: 37392283
-
hMSH2 coordinated with the expression of E2F1 promotes platinum response in epithelial ovarian cancer.J Int Med Res. 2023 Mar;51(3):3000605231163780. doi: 10.1177/03000605231163780. J Int Med Res. 2023. PMID: 36994850 Free PMC article.
-
Molecular mechanisms of cisplatin resistance in ovarian cancer.Genes Dis. 2023 Aug 2;11(6):101063. doi: 10.1016/j.gendis.2023.06.032. eCollection 2024 Nov. Genes Dis. 2023. PMID: 39224110 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

