Nucleosomal dsDNA Stimulates APOL1 Expression in Human Cultured Podocytes by Activating the cGAS/IFI16-STING Signaling Pathway
- PMID: 31664093
- PMCID: PMC6820523
- DOI: 10.1038/s41598-019-51998-w
Nucleosomal dsDNA Stimulates APOL1 Expression in Human Cultured Podocytes by Activating the cGAS/IFI16-STING Signaling Pathway
Abstract
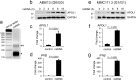
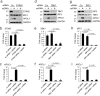
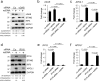
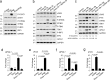
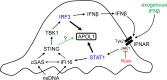
APOL1 alleles G1 and G2 are associated with faster progression to lupus nephritis (LN)-associated end-stage renal disease (LN-ESRD) in African Americans. Increased levels of type I interferons (IFNs) and nucleosome-associated double-stranded DNA (dsDNA) fragments (nsDNA) are the hallmark of this disease. Here, we identify cyclic GMP-AMP synthase (cGAS) and interferon-inducible protein 16 (IFI16) as the major DNA sensors in human immortalized podocytes. We also show that nsDNA triggers the expression of APOL1 and IFNβ via IRF3 activation through the cGAS/IFI16-STING pathway. We demonstrate that maximal APOL1 expression also requires the activation of type I IFN receptor (IFNAR) and STAT1 signaling triggered by IFNβ produced in response to nsDNA, or by exogenous IFNβ. Finally, we show that STAT1 activation is sufficient to upregulate IFI16, subsequently boosting APOL1 expression through a positive feedback mechanism. Collectively, we find that nsDNA-induced APOL1 expression is mediated by both IFNβ-independent and dependent signaling pathways triggered by activation of the cGAS/IFI16-STING pathway. We propose that simultaneous inhibition of STING and the IFNAR-STAT1 pathway may attenuate IFI16 expression, reduce IFI16-cGAS cross-talk, and prevent excessive APOL1 expression in human podocytes in response to nsDNA.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
IFI16 Is Indispensable for Promoting HIF-1α-Mediated APOL1 Expression in Human Podocytes under Hypoxic Conditions.Int J Mol Sci. 2024 Mar 15;25(6):3324. doi: 10.3390/ijms25063324. Int J Mol Sci. 2024. PMID: 38542298 Free PMC article.
-
Viral DNA Sensors IFI16 and Cyclic GMP-AMP Synthase Possess Distinct Functions in Regulating Viral Gene Expression, Immune Defenses, and Apoptotic Responses during Herpesvirus Infection.mBio. 2016 Nov 15;7(6):e01553-16. doi: 10.1128/mBio.01553-16. mBio. 2016. PMID: 27935834 Free PMC article.
-
IFI16 and cGAS cooperate in the activation of STING during DNA sensing in human keratinocytes.Nat Commun. 2017 Feb 13;8:14392. doi: 10.1038/ncomms14392. Nat Commun. 2017. PMID: 28194029 Free PMC article.
-
The cGAS-STING Pathway in Bacterial Infection and Bacterial Immunity.Front Immunol. 2022 Jan 13;12:814709. doi: 10.3389/fimmu.2021.814709. eCollection 2021. Front Immunol. 2022. PMID: 35095914 Free PMC article. Review.
-
cGAS-STING signaling pathway in intestinal homeostasis and diseases.Front Immunol. 2023 Sep 14;14:1239142. doi: 10.3389/fimmu.2023.1239142. eCollection 2023. Front Immunol. 2023. PMID: 37781354 Free PMC article. Review.
Cited by
-
cGAS‒STING signaling and function in metabolism and kidney diseases.J Mol Cell Biol. 2021 Dec 30;13(10):728-738. doi: 10.1093/jmcb/mjab066. J Mol Cell Biol. 2021. PMID: 34665236 Free PMC article. Review.
-
Role of the Innate Immune Response in Glomerular Disease Pathogenesis: Focus on Podocytes.Cells. 2024 Jul 6;13(13):1157. doi: 10.3390/cells13131157. Cells. 2024. PMID: 38995008 Free PMC article. Review.
-
Transcriptomic Analysis of Human Podocytes In Vitro: Effects of Differentiation and APOL1 Genotype.Kidney Int Rep. 2022 Oct 17;8(1):164-178. doi: 10.1016/j.ekir.2022.10.011. eCollection 2023 Jan. Kidney Int Rep. 2022. PMID: 36644347 Free PMC article.
-
ADAR regulates APOL1 via A-to-I RNA editing by inhibition of MDA5 activation in a paradoxical biological circuit.Proc Natl Acad Sci U S A. 2022 Nov;119(44):e2210150119. doi: 10.1073/pnas.2210150119. Epub 2022 Oct 25. Proc Natl Acad Sci U S A. 2022. PMID: 36282916 Free PMC article.
-
The clinical significance of apolipoprotein L1 in head and neck squamous cell carcinoma.Oncol Lett. 2020 Dec;20(6):377. doi: 10.3892/ol.2020.12240. Epub 2020 Oct 22. Oncol Lett. 2020. PMID: 33154775 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

