IGF-1 Inhibits Apoptosis of Porcine Primary Granulosa Cell by Targeting Degradation of BimEL
- PMID: 31661816
- PMCID: PMC6861984
- DOI: 10.3390/ijms20215356
IGF-1 Inhibits Apoptosis of Porcine Primary Granulosa Cell by Targeting Degradation of BimEL
Abstract
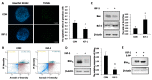
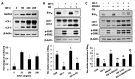
Insulin-like growth factor-1 (IGF-1) is an intra-ovarian growth factor that plays important endocrine or paracrine roles during ovarian development. IGF-1 affects ovarian function and female fertility through reducing apoptosis of granulosa cells, yet the underlying mechanism remains poorly characterized. Here, we aimed to address these knowledge gaps using porcine primary granulosa cells and examining the anti-apoptotic mechanisms of IGF-1. IGF-1 prevented the granulosa cell from apoptosis, as shown by TUNEL and Annexin V/PI detection, and gained the anti-apoptotic index, the ratio of Bcl-2/Bax. This process was partly mediated by reducing the pro-apoptotic BimEL (Bcl-2 Interacting Mediator of Cell Death-Extra Long) protein level. Western blotting showed that IGF-1 promoted BimEL phosphorylation through activating p-ERK1/2, and that the proteasome system was responsible for degradation of phosphorylated BimEL. Meanwhile, IGF-1 enhanced the Beclin1 level and the rate of LC3 II/LC3 I, indicating that autophagy was induced by IGF-1. By blocking the proteolysis processes of both proteasome and autophagy flux with MG132 and chloroquine, respectively, the BimEL did not reduce and the phosphorylated BimEL protein accumulated, thereby indicating that both proteasome and autophagy pathways were involved in the degradation of BimEL stimulated by IGF-1. In conclusion, IGF-1 inhibited porcine primary granulosa cell apoptosis via degradation of pro-apoptotic BimEL. This study is critical for us to further understand the mechanisms of follicular survival and atresia regulated by IGF-1. Moreover, it provides a direction for the treatment of infertility caused by ovarian dysplasia, such as polycystic ovary syndrome and the improvement of assisted reproductive technology.
Keywords: BimEL; IGF-1; apoptosis; autophagy; phosphorylation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Melatonin Promotes Ubiquitination of Phosphorylated Pro-Apoptotic Protein Bcl-2-Interacting Mediator of Cell Death-Extra Long (BimEL) in Porcine Granulosa Cells.Int J Mol Sci. 2018 Nov 1;19(11):3431. doi: 10.3390/ijms19113431. Int J Mol Sci. 2018. PMID: 30388852 Free PMC article.
-
Insulin mitigates apoptosis of porcine follicular granulosa cells by downregulating BimEL.Reprod Biol. 2019 Sep;19(3):293-298. doi: 10.1016/j.repbio.2019.08.003. Epub 2019 Sep 24. Reprod Biol. 2019. PMID: 31561987
-
Dynamics and Regulations of BimEL Ser65 and Thr112 Phosphorylation in Porcine Granulosa Cells during Follicular Atresia.Cells. 2020 Feb 10;9(2):402. doi: 10.3390/cells9020402. Cells. 2020. PMID: 32050589 Free PMC article.
-
Regulation mechanism of selective atresia in porcine follicles: regulation of granulosa cell apoptosis during atresia.J Reprod Dev. 2004 Oct;50(5):493-514. doi: 10.1262/jrd.50.493. J Reprod Dev. 2004. PMID: 15514456 Review.
-
Impaired autophagy and APP processing in Alzheimer's disease: The potential role of Beclin 1 interactome.Prog Neurobiol. 2013 Jul-Aug;106-107:33-54. doi: 10.1016/j.pneurobio.2013.06.002. Epub 2013 Jul 1. Prog Neurobiol. 2013. PMID: 23827971 Review.
Cited by
-
The Photoperiod Regulates Granulosa Cell Apoptosis through the FSH-Nodal/ALK7 Signaling Pathway in Phodopus sungorus.Animals (Basel). 2022 Dec 16;12(24):3570. doi: 10.3390/ani12243570. Animals (Basel). 2022. PMID: 36552491 Free PMC article.
-
Acupuncture Combined with Bushen-Jianpi Decoction Ameliorates the Ovarian Function of Diminished Ovarian Reserve Rats by Regulating Phosphoinositide 3-Kinase/AKT Signaling.Comb Chem High Throughput Screen. 2024;27(16):2402-2418. doi: 10.2174/0113862073264971231113061204. Comb Chem High Throughput Screen. 2024. PMID: 38178681
-
Characterization of polymorphisms in the follicle-stimulating hormone receptor and insulin-like growth factor-1 genes and their association with fertility traits in Jawa-Brebes cows.Vet World. 2023 Apr;16(4):711-716. doi: 10.14202/vetworld.2023.711-716. Epub 2023 Apr 9. Vet World. 2023. PMID: 37235159 Free PMC article.
-
IGF-1 as a Potential Therapy for Spinocerebellar Ataxia Type 3.Biomedicines. 2022 Feb 21;10(2):505. doi: 10.3390/biomedicines10020505. Biomedicines. 2022. PMID: 35203722 Free PMC article.
-
The impact of Apis dorsata forest honey administration on follicle-stimulating hormone and luteinizing hormone levels in rats (Rattus norvegicus) forced swim test as a chronic physical animal model.Open Vet J. 2024 Feb;14(2):738-742. doi: 10.5455/OVJ.2024.v14.i2.14. Epub 2024 Feb 29. Open Vet J. 2024. PMID: 38549577 Free PMC article.
References
-
- Jones J.I. Insulin-like growth factors and their binding proteins: Biological actions. Endocr. Rev. 1995;16:3–34. - PubMed
-
- Osada R., Ohshima H., Ishihara H., Yudoh K., Sakai K., Matsui H., Tsuji H. Autocrine/paracrine mechanism of insulin-like growth factor-1 secretion, and the effect of insulin-like growth factor-1 on proteoglycan synthesis in bovine intervertebral discs. J. Orthopaed Res. 1996;14:690–699. doi: 10.1002/jor.1100140503. - DOI - PubMed
-
- Charlton S.T., Cameron B.J., Glimm D.R., Foxcroft G.R., Kennelly J.J. Insulin-Like Growth Factor-I (Igf-1) Gene-Expression in Porcine Ovarian Tissue. Can. J. Anim. Sci. 1993;73:253–257. doi: 10.4141/cjas93-027. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

