Embryonic stem cell-derived extracellular vesicles enhance the therapeutic effect of mesenchymal stem cells
- PMID: 31660081
- PMCID: PMC6815953
- DOI: 10.7150/thno.35305
Embryonic stem cell-derived extracellular vesicles enhance the therapeutic effect of mesenchymal stem cells
Abstract
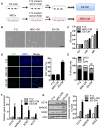
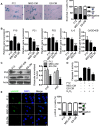
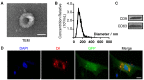
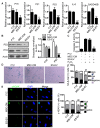
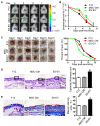
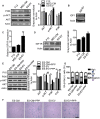
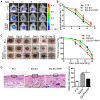
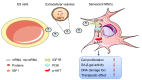
Background: Embryonic stem cells (ES) have a great potential for cell-based therapies in a regenerative medicine. However, the ethical and safety issues limit its clinical application. ES-derived extracellular vesicles (ES-EVs) have been reported suppress cellular senescence. Mesenchymal stem cells (MSCs) are widely used for clinical cell therapy. In this study, we investigated the beneficial effects of ES-EVs on aging MSCs to further enhancing their therapeutic effects. Methods:In vitro, we explored the rejuvenating effects of ES-EVs on senescent MSCs by senescence-associated β-gal (SA-β-gal) staining, immunostaining, and DNA damage foci analysis. The therapeutic effect of senescent MSC pre-treated with ES-EVs was also evaluated by using mouse cutaneous wound model. Results: We found that ES-EVs significantly rejuvenated the senescent MSCs in vitro and improve the therapeutic effects of MSCs in a mouse cutaneous wound model. In addition, we also identified that the IGF1/PI3K/AKT pathway mediated the antisenescence effects of ES-EVs on MSCs. Conclusions: Our results suggested that ES cells derived-extracellular vesicles possess the antisenescence properties, which significantly rejuvenate the senescent MSCs and enhance the therapeutic effects of MSCs. This strategy might emerge as a novel therapeutic strategy for MSCs clinical application.
Keywords: Cellular senescence; Embryonic stem cells; Extracellular vesicles; IGF1/PI3K/AKT pathway; Mesenchymal stem cells.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
Similar articles
-
Mesenchymal stem cell-derived extracellular vesicles reduce senescence and extend health span in mouse models of aging.Aging Cell. 2021 Apr;20(4):e13337. doi: 10.1111/acel.13337. Epub 2021 Mar 16. Aging Cell. 2021. PMID: 33728821 Free PMC article.
-
Clusterin-carrying extracellular vesicles derived from human umbilical cord mesenchymal stem cells restore the ovarian function of premature ovarian failure mice through activating the PI3K/AKT pathway.Stem Cell Res Ther. 2024 Sep 13;15(1):300. doi: 10.1186/s13287-024-03926-7. Stem Cell Res Ther. 2024. PMID: 39272156 Free PMC article.
-
Extracellular vesicles derived from human embryonic stem cell-MSCs ameliorate cirrhosis in thioacetamide-induced chronic liver injury.J Cell Physiol. 2018 Dec;233(12):9330-9344. doi: 10.1002/jcp.26413. Epub 2018 Jun 19. J Cell Physiol. 2018. PMID: 29266258
-
Therapeutic potential of mesenchymal stem cell-derived extracellular vesicles as novel cell-free therapy for treatment of autoimmune disorders.Exp Mol Pathol. 2021 Feb;118:104566. doi: 10.1016/j.yexmp.2020.104566. Epub 2020 Nov 6. Exp Mol Pathol. 2021. PMID: 33160961 Review.
-
Mesenchymal stromal cell-derived extracellular vesicles: regenerative and immunomodulatory effects and potential applications in sepsis.Cell Tissue Res. 2018 Oct;374(1):1-15. doi: 10.1007/s00441-018-2871-5. Epub 2018 Jun 28. Cell Tissue Res. 2018. PMID: 29955951 Review.
Cited by
-
Young small extracellular vesicles rejuvenate replicative senescence by remodeling Drp1 translocation-mediated mitochondrial dynamics.J Nanobiotechnology. 2024 Sep 5;22(1):543. doi: 10.1186/s12951-024-02818-5. J Nanobiotechnology. 2024. PMID: 39238005 Free PMC article.
-
Extracellular Vesicle-Based Therapeutics in Neurological Disorders.Pharmaceutics. 2022 Nov 30;14(12):2652. doi: 10.3390/pharmaceutics14122652. Pharmaceutics. 2022. PMID: 36559145 Free PMC article. Review.
-
Secretome of Stem Cells: Roles of Extracellular Vesicles in Diseases, Stemness, Differentiation, and Reprogramming.Tissue Eng Regen Med. 2022 Feb;19(1):19-33. doi: 10.1007/s13770-021-00406-4. Epub 2021 Nov 24. Tissue Eng Regen Med. 2022. PMID: 34817808 Free PMC article. Review.
-
Epigenetic Clock and Circadian Rhythms in Stem Cell Aging and Rejuvenation.J Pers Med. 2021 Oct 20;11(11):1050. doi: 10.3390/jpm11111050. J Pers Med. 2021. PMID: 34834402 Free PMC article. Review.
-
Tumorigenic Aspects of MSC Senescence-Implication in Cancer Development and Therapy.J Pers Med. 2021 Nov 2;11(11):1133. doi: 10.3390/jpm11111133. J Pers Med. 2021. PMID: 34834485 Free PMC article. Review.
References
-
- Gu W, Hong X, Potter C, Qu A, Xu Q. Mesenchymal stem cells and vascular regeneration. Microcirculation. 2017;24:e12324. - PubMed
-
- Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648–59. - PubMed
-
- Campisi J. The biology of replicative senescence. Eur J Cancer. 1997;33:703–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

