Active roles of dysfunctional vascular endothelium in fibrosis and cancer
- PMID: 31656195
- PMCID: PMC6816223
- DOI: 10.1186/s12929-019-0580-3
Active roles of dysfunctional vascular endothelium in fibrosis and cancer
Abstract
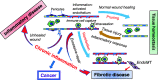
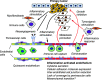
Chronic inflammation is the underlying pathological condition that results in fibrotic diseases. More recently, many forms of cancer have also been linked to chronic tissue inflammation. While stromal immune cells and myofibroblasts have been recognized as major contributors of cytokines and growth factors that foster the formation of fibrotic tissue, the endothelium has traditionally been regarded as a passive player in the pathogenic process, or even as a barrier since it provides a physical divide between the circulating immune cells and the inflamed tissues. Recent findings, however, have indicated that endothelial cells in fact play a crucial role in the inflammatory response. Endothelial cells can be activated by cytokine signaling and express inflammatory markers, which can sustain or exacerbate the inflammatory process. For example, the activated endothelium can recruit and activate leukocytes, thus perpetuating tissue inflammation, while sustained stimulation of endothelial cells may lead to endothelial-to-mesenchymal transition that contributes to fibrosis. Since chronic inflammation has now been recognized as a significant contributing factor to tumorigenesis, it has also emerged that activation of endothelium also occurs in the tumor microenvironment. This review summarizes recent findings characterizing the molecular and cellular changes in the vascular endothelium that contribute to tissue fibrosis, and potentially to cancer formation.
Keywords: Cancer vasculature; Endothelial cells; Fibrosis; Inflammation.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Endothelial-to-mesenchymal transition: Cytokine-mediated pathways that determine endothelial fibrosis under inflammatory conditions.Cytokine Growth Factor Rev. 2017 Feb;33:41-54. doi: 10.1016/j.cytogfr.2016.09.002. Epub 2016 Sep 23. Cytokine Growth Factor Rev. 2017. PMID: 27692608 Review.
-
Does transformation of microvascular endothelial cells into myofibroblasts play a key role in the etiology and pathology of fibrotic disease?Med Hypotheses. 2007;68(3):650-5. doi: 10.1016/j.mehy.2006.07.053. Epub 2006 Oct 12. Med Hypotheses. 2007. PMID: 17045756
-
NLRP3 Inflammasome Activation Contributes to Mechanical Stretch-Induced Endothelial-Mesenchymal Transition and Pulmonary Fibrosis.Crit Care Med. 2018 Jan;46(1):e49-e58. doi: 10.1097/CCM.0000000000002799. Crit Care Med. 2018. PMID: 29088003
-
The Role of Endothelial Dysfunction and Inflammation in Chronic Venous Disease.Ann Vasc Surg. 2018 Jan;46:380-393. doi: 10.1016/j.avsg.2017.06.131. Epub 2017 Jul 6. Ann Vasc Surg. 2018. PMID: 28688874 Review.
-
Vascular endothelium--role in chronic inflammatory disease.Postepy Biochem. 2013;59(4):415-23. Postepy Biochem. 2013. PMID: 24745172 Review.
Cited by
-
Inflammatory stress-mediated chromatin changes underlie dysfunction in endothelial cells.bioRxiv [Preprint]. 2023 Oct 16:2023.10.11.561959. doi: 10.1101/2023.10.11.561959. bioRxiv. 2023. PMID: 37905100 Free PMC article. Preprint.
-
WRN Nuclease-Mediated EcDNA Clearance Enhances Antitumor Therapy in Conjunction with Trehalose Dimycolate/Mesoporous Silica Nanoparticles.Adv Sci (Weinh). 2024 Oct;11(40):e2407026. doi: 10.1002/advs.202407026. Epub 2024 Aug 29. Adv Sci (Weinh). 2024. PMID: 39206698 Free PMC article.
-
Enhancement of Wound Healing Efficacy by Chitosan-based Hydrocolloid on Sprague Dawley Rats.In Vivo. 2023 May-Jun;37(3):1052-1064. doi: 10.21873/invivo.13180. In Vivo. 2023. PMID: 37103063 Free PMC article.
-
Atherosclerosis and the Bidirectional Relationship between Cancer and Cardiovascular Disease: From Bench to Bedside-Part 1.Int J Mol Sci. 2024 Apr 11;25(8):4232. doi: 10.3390/ijms25084232. Int J Mol Sci. 2024. PMID: 38673815 Free PMC article. Review.
-
Arterial Hypertension and Plasma Glucose Modulate the Vasoactive Effects of Nitroso-Sulfide Coupled Signaling in Human Intrarenal Arteries.Molecules. 2020 Jun 23;25(12):2886. doi: 10.3390/molecules25122886. Molecules. 2020. PMID: 32585916 Free PMC article.
References
-
- Eming SA, Koch M, Krieger A, Brachvogel B, Kreft S, Bruckner-Tuderman L, et al. Differential proteomic analysis distinguishes tissue repair biomarker signatures in wound exudates obtained from normal healing and chronic wounds. J Proteome Res. 2010;9(9):4758–4766. doi: 10.1021/pr100456d. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

