RARβ Agonist Drug (C286) Demonstrates Efficacy in a Pre-clinical Neuropathic Pain Model Restoring Multiple Pathways via DNA Repair Mechanisms
- PMID: 31655065
- PMCID: PMC6833472
- DOI: 10.1016/j.isci.2019.09.020
RARβ Agonist Drug (C286) Demonstrates Efficacy in a Pre-clinical Neuropathic Pain Model Restoring Multiple Pathways via DNA Repair Mechanisms
Erratum in
-
RARβ Agonist Drug (C286) Demonstrates Efficacy in a Pre-clinical Neuropathic Pain Model Restoring Multiple Pathways via DNA Repair Mechanisms.iScience. 2019 Nov 22;21:562-563. doi: 10.1016/j.isci.2019.10.068. Epub 2019 Nov 11. iScience. 2019. PMID: 31726373 Free PMC article. No abstract available.
Abstract
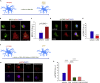
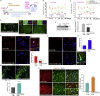
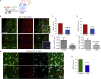
Neuropathic pain (NP) is associated with profound gene expression alterations within the nociceptive system. DNA mechanisms, such as epigenetic remodeling and repair pathways have been implicated in NP. Here we have used a rat model of peripheral nerve injury to study the effect of a recently developed RARβ agonist, C286, currently under clinical research, in NP. A 4-week treatment initiated 2 days after the injury normalized pain sensation. Genome-wide and pathway enrichment analysis showed that multiple mechanisms persistently altered in the spinal cord were restored to preinjury levels by the agonist. Concomitant upregulation of DNA repair proteins, ATM and BRCA1, the latter being required for C286-mediated pain modulation, suggests that early DNA repair may be important to prevent phenotypic epigenetic imprints in NP. Thus, C286 is a promising drug candidate for neuropathic pain and DNA repair mechanisms may be useful therapeutic targets to explore.
Keywords: Biological Sciences; Neuroscience; Transcriptomics.
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interests. C286 synthesis and use in nerve injuries and neuropathic pain is protected under patents (PCT/EP2015/08,002; PCT/EP2017/0604802; GB1907647).
Figures


Similar articles
-
RARβ Agonist Drug (C286) Demonstrates Efficacy in a Pre-clinical Neuropathic Pain Model Restoring Multiple Pathways via DNA Repair Mechanisms.iScience. 2019 Nov 22;21:562-563. doi: 10.1016/j.isci.2019.10.068. Epub 2019 Nov 11. iScience. 2019. PMID: 31726373 Free PMC article. No abstract available.
-
C286, an orally available retinoic acid receptor β agonist drug, regulates multiple pathways to achieve spinal cord injury repair.Front Mol Neurosci. 2024 Aug 20;17:1411384. doi: 10.3389/fnmol.2024.1411384. eCollection 2024. Front Mol Neurosci. 2024. PMID: 39228795 Free PMC article.
-
Regulation of Myelination by Exosome Associated Retinoic Acid Release from NG2-Positive Cells.J Neurosci. 2019 Apr 17;39(16):3013-3027. doi: 10.1523/JNEUROSCI.2922-18.2019. Epub 2019 Feb 13. J Neurosci. 2019. PMID: 30760627 Free PMC article.
-
Functional roles of lncRNAs and its potential mechanisms in neuropathic pain.Clin Epigenetics. 2019 May 15;11(1):78. doi: 10.1186/s13148-019-0671-8. Clin Epigenetics. 2019. PMID: 31092294 Free PMC article. Review.
-
Bioinformatics Genes and Pathway Analysis for Chronic Neuropathic Pain after Spinal Cord Injury.Biomed Res Int. 2017;2017:6423021. doi: 10.1155/2017/6423021. Epub 2017 Oct 15. Biomed Res Int. 2017. PMID: 29164149 Free PMC article. Review.
Cited by
-
Synthetic Retinoids Beyond Cancer Therapy.Annu Rev Pharmacol Toxicol. 2022 Jan 6;62:155-175. doi: 10.1146/annurev-pharmtox-052120-104428. Epub 2021 Sep 13. Annu Rev Pharmacol Toxicol. 2022. PMID: 34516292 Free PMC article. Review.
-
Recent advances in the design of RAR α and RAR β agonists as orally bioavailable drugs. A review.Bioorg Med Chem. 2020 Oct 15;28(20):115664. doi: 10.1016/j.bmc.2020.115664. Epub 2020 Jul 29. Bioorg Med Chem. 2020. PMID: 33069074 Free PMC article. Review.
-
Revisiting APP secretases: an overview on the holistic effects of retinoic acid receptor stimulation in APP processing.Cell Mol Life Sci. 2022 Jan 28;79(2):101. doi: 10.1007/s00018-021-04090-4. Cell Mol Life Sci. 2022. PMID: 35089425 Free PMC article. Review.
References
-
- Bar-Shira A., Rashi-Elkeles S., Zlochover L., Moyal L., Smorodinsky N.I., Seger R., Shiloh Y. ATM-dependent activation of the gene encoding MAP kinase phosphatase 5 by radiomimetic DNA damage. Oncogene. 2002;21:849–855. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

