Inflammation in Renal Diseases: New and Old Players
- PMID: 31649546
- PMCID: PMC6792167
- DOI: 10.3389/fphar.2019.01192
Inflammation in Renal Diseases: New and Old Players
Abstract
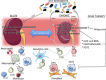
Inflammation, a process intimately linked to renal disease, can be defined as a complex network of interactions between renal parenchymal cells and resident immune cells, such as macrophages and dendritic cells, coupled with recruitment of circulating monocytes, lymphocytes, and neutrophils. Once stimulated, these cells activate specialized structures such as Toll-like receptor and Nod-like receptor (NLR). By detecting danger-associated molecules, these receptors can set in motion major innate immunity pathways such as nuclear factor ĸB (NF-ĸB) and NLRP3 inflammasome, causing metabolic reprogramming and phenotype changes of immune and parenchymal cells and triggering the secretion of a number of inflammatory mediators that can cause irreversible tissue damage and functional loss. Growing evidence suggests that this response can be deeply impacted by the crosstalk between the kidneys and other organs, such as the gut. Changes in the composition and/or metabolite production of the gut microbiota can influence inflammation, oxidative stress, and fibrosis, thus offering opportunities to positively manipulate the composition and/or functionality of gut microbiota and, consequentially, ameliorate deleterious consequences of renal diseases. In this review, we summarize the most recent evidence that renal inflammation can be ameliorated by interfering with the gut microbiota through the administration of probiotics, prebiotics, and postbiotics. In addition to these innovative approaches, we address the recent discovery of new targets for drugs long in use in clinical practice. Angiotensin II receptor antagonists, NF-ĸB inhibitors, thiazide diuretics, and antimetabolic drugs can reduce renal macrophage infiltration and slow down the progression of renal disease by mechanisms independent of those usually attributed to these compounds. Allopurinol, an inhibitor of uric acid production, has been shown to decrease renal inflammation by limiting activation of the NLRP3 inflammasome. So far, these protective effects have been shown in experimental studies only. Clinical studies will establish whether these novel strategies can be incorporated into the arsenal of treatments intended to prevent the progression of human disease.
Keywords: NF- kappa B; acute kidney injury (AKI); chronic kidney disease (CKD); gut microbiota; inflammation.
Copyright © 2019 Andrade-Oliveira, Foresto-Neto, Watanabe, Zatz and Câmara.
Figures

Similar articles
-
Pathogenic role of innate immunity in a model of chronic NO inhibition associated with salt overload.Am J Physiol Renal Physiol. 2019 Oct 1;317(4):F1058-F1067. doi: 10.1152/ajprenal.00251.2019. Epub 2019 Aug 14. Am J Physiol Renal Physiol. 2019. PMID: 31411073
-
Hyperuricemia-induced NLRP3 activation of macrophages contributes to the progression of diabetic nephropathy.Am J Physiol Renal Physiol. 2015 May 1;308(9):F993-F1003. doi: 10.1152/ajprenal.00637.2014. Epub 2015 Jan 28. Am J Physiol Renal Physiol. 2015. PMID: 25651569
-
Simultaneous activation of innate and adaptive immunity participates in the development of renal injury in a model of heavy proteinuria.Biosci Rep. 2018 Jul 12;38(4):BSR20180762. doi: 10.1042/BSR20180762. Print 2018 Aug 31. Biosci Rep. 2018. PMID: 29914975 Free PMC article.
-
The nuclear factor kappa B signaling pathway is a master regulator of renal fibrosis.Front Pharmacol. 2024 Jan 16;14:1335094. doi: 10.3389/fphar.2023.1335094. eCollection 2023. Front Pharmacol. 2024. PMID: 38293668 Free PMC article. Review.
-
The Microbiota-Inflammasome Hypothesis of Major Depression.Bioessays. 2018 Sep;40(9):e1800027. doi: 10.1002/bies.201800027. Epub 2018 Jul 13. Bioessays. 2018. PMID: 30004130 Review.
Cited by
-
Friend or Foe? An Unrecognized Role of Uric Acid in Cancer Development and the Potential Anticancer Effects of Uric Acid-lowering Drugs.J Cancer. 2020 Jul 6;11(17):5236-5244. doi: 10.7150/jca.46200. eCollection 2020. J Cancer. 2020. PMID: 32742469 Free PMC article. Review.
-
Identification of immune-related molecular clusters and diagnostic markers in chronic kidney disease based on cluster analysis.Front Genet. 2023 Feb 6;14:1111976. doi: 10.3389/fgene.2023.1111976. eCollection 2023. Front Genet. 2023. PMID: 36814902 Free PMC article.
-
Association Between Salivary Cytokines, Chemokines and Growth Factors and Salivary Gland Function in Children with Chronic Kidney Disease.J Inflamm Res. 2023 Mar 14;16:1103-1120. doi: 10.2147/JIR.S399786. eCollection 2023. J Inflamm Res. 2023. PMID: 36941986 Free PMC article.
-
Annexin A2 (ANXA2) regulates the transcription and alternative splicing of inflammatory genes in renal tubular epithelial cells.BMC Genomics. 2022 Jul 29;23(1):544. doi: 10.1186/s12864-022-08748-6. BMC Genomics. 2022. PMID: 35906541 Free PMC article.
-
The Emerging Role of Innate Immunity in Chronic Kidney Diseases.Int J Mol Sci. 2020 Jun 4;21(11):4018. doi: 10.3390/ijms21114018. Int J Mol Sci. 2020. PMID: 32512831 Free PMC article. Review.
References
-
- Aguilar-Toala J. E., Garcia-Valera H., Garcia H. S., Mata-Haro V., Gonzalez-Cordova A. F., Vallejo-Cordoba B., et al. (2018). Postbiotics: an evolving term within the functional foods field. Trends Food Sci. Technol. 75, 105–114. 10.1016/j.tifs.2018.03.009 - DOI
Publication types
LinkOut - more resources
Full Text Sources

