Membrane-bound TNF mediates microtubule-targeting chemotherapeutics-induced cancer cytolysis via juxtacrine inter-cancer-cell death signaling
- PMID: 31645676
- PMCID: PMC7206059
- DOI: 10.1038/s41418-019-0441-3
Membrane-bound TNF mediates microtubule-targeting chemotherapeutics-induced cancer cytolysis via juxtacrine inter-cancer-cell death signaling
Abstract
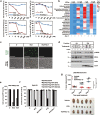
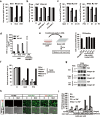
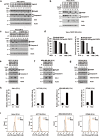
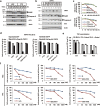
Microtubule-targeting agents (MTAs) are a class of most widely used chemotherapeutics and their mechanism of action has long been assumed to be mitotic arrest of rapidly dividing tumor cells. In contrast to such notion, here we show-in many cancer cell types-MTAs function by triggering membrane TNF (memTNF)-mediated cancer-cell-to-cancer-cell killing, which differs greatly from other non-MTA cell-cycle-arresting agents. The killing is through programmed cell death (PCD), either in way of necroptosis when RIP3 kinase is expressed, or of apoptosis in its absence. Mechanistically, MTAs induce memTNF transcription via the JNK-cJun signaling pathway. With respect to chemotherapy regimens, our results establish that memTNF-mediated killing is significantly augmented by IAP antagonists (Smac mimetics) in a broad spectrum of cancer types, and with their effects most prominently manifested in patient-derived xenograft (PDX) models in which cell-cell contacts are highly reminiscent of human tumors. Therefore, our finding indicates that memTNF can serve as a marker for patient responsiveness, and Smac mimetics will be effective adjuvants for MTA chemotherapeutics. The present study reframes our fundamental biochemical understanding of how MTAs take advantage of the natural tight contact of tumor cells and utilize memTNF-mediated death signaling to induce the entire tumor regression.
Conflict of interest statement
Shanghai Institute of Biochemistry and Cell Biology is in the process of applying for a patent application covering the use of paclitaxel and IAP inhibitors to treat breast cancers that lists JZ and LS as inventors (CN 201811388749.2).
Figures


Similar articles
-
Smac mimetics and type II interferon synergistically induce necroptosis in various cancer cell lines.Cancer Lett. 2017 Dec 1;410:228-237. doi: 10.1016/j.canlet.2017.09.002. Epub 2017 Sep 18. Cancer Lett. 2017. PMID: 28923396
-
Smac mimetic triggers necroptosis in pancreatic carcinoma cells when caspase activation is blocked.Cancer Lett. 2016 Sep 28;380(1):31-8. doi: 10.1016/j.canlet.2016.05.036. Epub 2016 Jun 3. Cancer Lett. 2016. PMID: 27267809
-
Emodin induced necroptosis in the glioma cell line U251 via the TNF-α/RIP1/RIP3 pathway.Invest New Drugs. 2020 Feb;38(1):50-59. doi: 10.1007/s10637-019-00764-w. Epub 2019 Mar 28. Invest New Drugs. 2020. PMID: 30924024 Free PMC article.
-
Interphase microtubules: chief casualties in the war on cancer?Drug Discov Today. 2014 Jul;19(7):824-9. doi: 10.1016/j.drudis.2013.10.022. Epub 2013 Nov 4. Drug Discov Today. 2014. PMID: 24201225 Free PMC article. Review.
-
Killing a cancer: what are the alternatives?Nat Rev Cancer. 2012 May 11;12(6):411-24. doi: 10.1038/nrc3264. Nat Rev Cancer. 2012. PMID: 22576162 Review.
Cited by
-
HSPA8 inhibitors augment cancer chemotherapeutic effectiveness via potentiating necroptosis.Mol Biol Cell. 2024 Aug 1;35(8):ar108. doi: 10.1091/mbc.E24-04-0194. Epub 2024 Jul 3. Mol Biol Cell. 2024. PMID: 38959101 Free PMC article.
-
Unveiling the landscape of cytokine research in glioma immunotherapy: a scientometrics analysis.Front Pharmacol. 2024 Jan 8;14:1333124. doi: 10.3389/fphar.2023.1333124. eCollection 2023. Front Pharmacol. 2024. PMID: 38259287 Free PMC article. Review.
-
Ccl3 enhances docetaxel chemosensitivity in breast cancer by triggering proinflammatory macrophage polarization.J Immunother Cancer. 2022 May;10(5):e003793. doi: 10.1136/jitc-2021-003793. J Immunother Cancer. 2022. PMID: 35613826 Free PMC article.
-
miR-4721, Induced by EBV-miR-BART22, Targets GSK3β to Enhance the Tumorigenic Capacity of NPC through the WNT/β-catenin Pathway.Mol Ther Nucleic Acids. 2020 Sep 23;22:557-571. doi: 10.1016/j.omtn.2020.09.021. eCollection 2020 Dec 4. Mol Ther Nucleic Acids. 2020. PMID: 33230457 Free PMC article.
-
Apoptotic dysregulation mediates stem cell competition and tissue regeneration.Nat Commun. 2023 Nov 20;14(1):7547. doi: 10.1038/s41467-023-41684-x. Nat Commun. 2023. PMID: 37985759 Free PMC article.
References
-
- Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4:253–65. - PubMed
-
- Castedo M, Perfettini JL, Roumier T, Andreau K, Medema R, Kroemer G. Cell death by mitotic catastrophe: a molecular definition. Oncogene. 2004;23:2825–37. - PubMed
-
- Rieder CL, Maiato H. Stuck in division or passing through: what happens when cells cannot satisfy the spindle assembly checkpoint. Dev Cell. 2004;7:637–51. - PubMed
-
- Weaver BA, Cleveland DW. Decoding the links between mitosis, cancer, and chemotherapy: the mitotic checkpoint, adaptation, and cell death. Cancer Cell. 2005;8:7–12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

