Withaferin A triggers G2/M arrest and intrinsic apoptosis in glioblastoma cells via ATF4-ATF3-CHOP axis
- PMID: 31642559
- PMCID: PMC6985693
- DOI: 10.1111/cpr.12706
Withaferin A triggers G2/M arrest and intrinsic apoptosis in glioblastoma cells via ATF4-ATF3-CHOP axis
Erratum in
-
Correction to "Withaferin A triggers G2/M arrest and intrinsic apoptosis in glioblastoma cells via ATF4-ATF3-CHOP axis".Cell Prolif. 2024 Oct;57(10):e13706. doi: 10.1111/cpr.13706. Epub 2024 Jun 26. Cell Prolif. 2024. PMID: 38926944 Free PMC article. No abstract available.
Abstract
Objective: Withaferin A (WA) is a bioactive compound with a remarkable anti-cancer effect derived from Withania somnifera, commonly known as ashwagandha. However, the anti-cancer mechanisms of WA in glioblastoma multiforme (GBM) are still unclear.
Materials and methods: Cell viability assays and xenografted nude mice were used to evaluate the effects of WA, along with flow cytometry to detect apoptosis and cell cycle of GBM. RNA-seq analysis, Western blotting, immunofluorescence staining, qRT-PCR and siRNA gene silencing were carried out to determine the signalling pathways affected by WA.
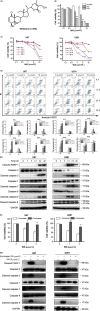
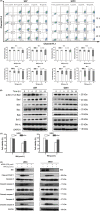
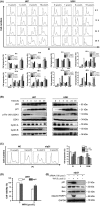
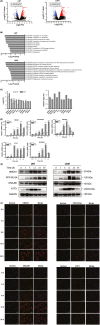
Results: Withaferin A significantly inhibited the growth of GBM in vitro and in vivo and triggered the intrinsic apoptosis of GBM cells by up-regulating expression of Bim and Bad. WA arrested GBM cells at the G2/M phase of the cell cycle through dephosphorylating Thr161 of CDK1 by activating p53-independent p21 up-regulation. Knockdown of p21 restored cell cycle progression and cell viability by down-regulating the expression of Bad rather than Bim. We demonstrated that endoplasmic reticulum (ER) stress induced by WA through the ATF4-ATF3-CHOP axis, initiated apoptosis and G2/M arrest in GBM cells.
Conclusion: We revealed a novel pathway that elucidated WA activation of apoptosis and G2/M arrest in GBM cells through the ATF4-ATF3-CHOP axis. This discovery is important for optimization of WA-based regimens for prevention and/or treatment of GBM.
Keywords: apoptosis; cell cycle; endoplasmic reticulum; glioblastoma; unfolded protein response.
© 2019 The Authors. Cell Proliferation Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest that pertain to this work.
Figures


Similar articles
-
Withaferin A, a natural thioredoxin reductase 1 (TrxR1) inhibitor, synergistically enhances the antitumor efficacy of sorafenib through ROS-mediated ER stress and DNA damage in hepatocellular carcinoma cells.Phytomedicine. 2024 Jun;128:155317. doi: 10.1016/j.phymed.2023.155317. Epub 2023 Dec 25. Phytomedicine. 2024. PMID: 38537439
-
KLF6 Induces Apoptosis in Human Lens Epithelial Cells Through the ATF4-ATF3-CHOP Axis.Drug Des Devel Ther. 2020 Mar 9;14:1041-1055. doi: 10.2147/DDDT.S218467. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 32210535 Free PMC article.
-
Targeting the ABC transporter ABCB5 sensitizes glioblastoma to temozolomide-induced apoptosis through a cell-cycle checkpoint regulation mechanism.J Biol Chem. 2020 May 29;295(22):7774-7788. doi: 10.1074/jbc.RA120.013778. Epub 2020 Apr 20. J Biol Chem. 2020. PMID: 32317280 Free PMC article.
-
Molecular targets and mechanisms of cancer prevention and treatment by withaferin a, a naturally occurring steroidal lactone.AAPS J. 2014 Jan;16(1):1-10. doi: 10.1208/s12248-013-9531-1. Epub 2013 Sep 18. AAPS J. 2014. PMID: 24046237 Free PMC article. Review.
-
Ashwagandha-Induced Programmed Cell Death in the Treatment of Breast Cancer.Curr Issues Mol Biol. 2024 Jul 18;46(7):7668-7685. doi: 10.3390/cimb46070454. Curr Issues Mol Biol. 2024. PMID: 39057095 Free PMC article. Review.
Cited by
-
ATF3 Promotes Arsenic-Induced Apoptosis and Oppositely Regulates DR5 and Bcl-xL Expression in Human Bronchial Epithelial Cells.Int J Mol Sci. 2021 Apr 19;22(8):4223. doi: 10.3390/ijms22084223. Int J Mol Sci. 2021. PMID: 33921748 Free PMC article.
-
Identification of an autophagy-related gene signature for predicting prognosis and immune activity in pancreatic adenocarcinoma.Sci Rep. 2022 Apr 29;12(1):7006. doi: 10.1038/s41598-022-11050-w. Sci Rep. 2022. PMID: 35488119 Free PMC article.
-
Identification of CDK1, PBK, and CHEK1 as an Oncogenic Signature in Glioblastoma: A Bioinformatics Approach to Repurpose Dapagliflozin as a Therapeutic Agent.Int J Mol Sci. 2023 Nov 16;24(22):16396. doi: 10.3390/ijms242216396. Int J Mol Sci. 2023. PMID: 38003585 Free PMC article.
-
TCA-phospholipid-glycolysis targeted triple therapy effectively suppresses ATP production and tumor growth in glioblastoma.Theranostics. 2022 Oct 3;12(16):7032-7050. doi: 10.7150/thno.74197. eCollection 2022. Theranostics. 2022. PMID: 36276638 Free PMC article.
-
Withaferin A Exerts Preventive Effect on Liver Fibrosis through Oxidative Stress Inhibition in a Sirtuin 3-Dependent Manner.Oxid Med Cell Longev. 2020 Sep 24;2020:2452848. doi: 10.1155/2020/2452848. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 33029279 Free PMC article.
References
-
- Wen PY, Reardon DA. Neuro‐oncology in 2015: progress in glioma diagnosis, classification and treatment. Nat Rev Neurol. 2016;12(2):69‐70. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987‐996. - PubMed
-
- Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492‐507. - PubMed
MeSH terms
Substances
Grants and funding
- 2018ZX09711001-005-025/Technology Major Projects for "Major New Drugs Innovation and Development"
- 81573454/National Natural Science Foundation of China
- 81703536/National Natural Science Foundation of China
- 81703565/National Natural Science Foundation of China
- 81803584/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

