An Overview of Saturated Cyclic Ethers: Biological Profiles and Synthetic Strategies
- PMID: 31640154
- PMCID: PMC6833478
- DOI: 10.3390/molecules24203778
An Overview of Saturated Cyclic Ethers: Biological Profiles and Synthetic Strategies
Abstract
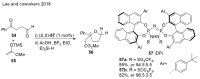
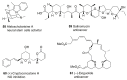
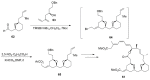
Saturated oxygen heterocycles are widely found in a broad array of natural products and other biologically active molecules. In medicinal chemistry, small and medium rings are also important synthetic intermediates since they can undergo ring-opening and -expansion reactions. These applications have driven numerous studies on the synthesis of oxygen-containing heterocycles and considerable effort has been devoted toward the development of methods for the construction of saturated oxygen heterocycles. This paper provides an overview of the biological roles and synthetic strategies of saturated cyclic ethers, covering some of the most studied and newly discovered related natural products in recent years. This paper also reports several promising and newly developed synthetic methods, emphasizing 3-7 membered rings.
Keywords: cyclic ethers; saturated oxygen heterocycles; total synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
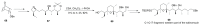
Similar articles
-
SmI(2)-induced reductive cyclizations for the synthesis of cyclic ethers and applications in natural product synthesis.Chem Soc Rev. 2010 Jun;39(6):1955-72. doi: 10.1039/b902737h. Epub 2010 Mar 23. Chem Soc Rev. 2010. PMID: 20502797 Review.
-
Total synthesis and complete structural assignment of gambieric acid A, a large polycyclic ether marine natural product.Chem Rec. 2014 Aug;14(4):678-703. doi: 10.1002/tcr.201402052. Epub 2014 Aug 4. Chem Rec. 2014. PMID: 25092231 Review.
-
Total Synthesis of the Marine Ladder Polyether Gymnocin B.J Am Chem Soc. 2019 Jul 17;141(28):11239-11244. doi: 10.1021/jacs.9b04696. Epub 2019 Jul 8. J Am Chem Soc. 2019. PMID: 31283211 Free PMC article.
-
Selected hybrid natural products as tubulin modulators.Eur J Med Chem. 2015 Apr 13;94:497-508. doi: 10.1016/j.ejmech.2014.10.062. Epub 2014 Oct 29. Eur J Med Chem. 2015. PMID: 25455639 Review.
-
[Reaction Development Utilizing the Features of Chemical Elements and Synthesis of Marine Natural Products].Yakugaku Zasshi. 2018;138(11):1335-1344. doi: 10.1248/yakushi.18-00126. Yakugaku Zasshi. 2018. PMID: 30381641 Review. Japanese.
Cited by
-
αβ,α'β'-Diepoxyketones are mechanism-based inhibitors of nucleophilic cysteine enzymes.Chem Commun (Camb). 2023 Oct 26;59(86):12859-12862. doi: 10.1039/d3cc02932h. Chem Commun (Camb). 2023. PMID: 37815791 Free PMC article.
-
Synthetic efforts on the road to marine natural products bearing 4-O-2,3,4,6-tetrasubstituted THPs: an update.RSC Adv. 2021 Feb 3;11(10):5832-5858. doi: 10.1039/d0ra10755g. eCollection 2021 Jan 28. RSC Adv. 2021. PMID: 35423108 Free PMC article. Review.
-
Enantioselective Hydroalkoxylation of 1,3-Dienes via Ni-Catalysis.J Am Chem Soc. 2023 Feb 10;145(7):3909-14. doi: 10.1021/jacs.2c12779. Online ahead of print. J Am Chem Soc. 2023. PMID: 36763788 Free PMC article.
-
Effect of Salinomycin on Expression Pattern of Genes Associated with Apoptosis in Endometrial Cancer Cell Line.Curr Pharm Biotechnol. 2020;21(12):1269-1277. doi: 10.2174/1389201021666200513074022. Curr Pharm Biotechnol. 2020. PMID: 32400328 Free PMC article.
-
On the application of 3d metals for C-H activation toward bioactive compounds: The key step for the synthesis of silver bullets.Beilstein J Org Chem. 2021 Jul 30;17:1849-1938. doi: 10.3762/bjoc.17.126. eCollection 2021. Beilstein J Org Chem. 2021. PMID: 34386103 Free PMC article. Review.
References
-
- Martín T., Padron J.I., Martin V.S. Strategies for the synthesis of cyclic ethers of marine natural products. Synlett. 2014;25:12–32. doi: 10.1055/s-0033-1340073. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

