The formation of perinucleolar bodies is important for normal leaf development and requires the zinc-finger DNA-binding motif in Arabidopsis ASYMMETRIC LEAVES2
- PMID: 31639235
- PMCID: PMC7155070
- DOI: 10.1111/tpj.14579
The formation of perinucleolar bodies is important for normal leaf development and requires the zinc-finger DNA-binding motif in Arabidopsis ASYMMETRIC LEAVES2
Abstract
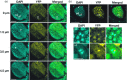
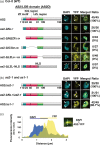
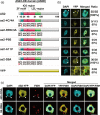
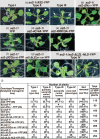
In Arabidopsis, the ASYMMETRIC LEAVES2 (AS2) protein plays a key role in the formation of flat symmetric leaves via direct repression of the abaxial gene ETT/ARF3. AS2 encodes a plant-specific nuclear protein that contains the AS2/LOB domain, which includes a zinc-finger (ZF) motif that is conserved in the AS2/LOB family. We have shown that AS2 binds to the coding DNA of ETT/ARF3, which requires the ZF motif. AS2 is co-localized with AS1 in perinucleolar bodies (AS2 bodies). To identify the amino acid signals in AS2 required for formation of AS2 bodies and function(s) in leaf formation, we constructed recombinant DNAs that encoded mutant AS2 proteins fused to yellow fluorescent protein. We examined the subcellular localization of these proteins in cells of cotyledons and leaf primordia of transgenic plants and cultured cells. The amino acid signals essential for formation of AS2 bodies were located within and adjacent to the ZF motif. Mutant AS2 that failed to form AS2 bodies also failed to rescue the as2-1 mutation. Our results suggest the importance of the formation of AS2 bodies and the nature of interactions of AS2 with its target DNA and nucleolar factors including NUCLEOLIN1. The partial overlap of AS2 bodies with perinucleolar chromocenters with condensed ribosomal RNA genes implies a correlation between AS2 bodies and the chromatin state. Patterns of AS2 bodies in cells during interphase and mitosis in leaf primordia were distinct from those in cultured cells, suggesting that the formation and distribution of AS2 bodies are developmentally modulated in plants.
Keywords: 45S ribosomal RNA genes; ETTIN/AUXIN RESEPONSE FACTOR3; chromocenter; epigenetic factor AS2; nucleolus; perinucleolar body; zinc-finger motif.
© 2019 The Authors. The Plant Journal published by Society for Experimental Biology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of financial interest.
Figures


Similar articles
-
Arabidopsis ASYMMETRIC LEAVES2 and Nucleolar Factors Are Coordinately Involved in the Perinucleolar Patterning of AS2 Bodies and Leaf Development.Plants (Basel). 2023 Oct 19;12(20):3621. doi: 10.3390/plants12203621. Plants (Basel). 2023. PMID: 37896084 Free PMC article.
-
Roles of ASYMMETRIC LEAVES2 (AS2) and Nucleolar Proteins in the Adaxial-Abaxial Polarity Specification at the Perinucleolar Region in Arabidopsis.Int J Mol Sci. 2020 Oct 3;21(19):7314. doi: 10.3390/ijms21197314. Int J Mol Sci. 2020. PMID: 33022996 Free PMC article. Review.
-
Arabidopsis ASYMMETRIC LEAVES2 (AS2): roles in plant morphogenesis, cell division, and pathogenesis.J Plant Res. 2022 Jan;135(1):3-14. doi: 10.1007/s10265-021-01349-6. Epub 2021 Oct 19. J Plant Res. 2022. PMID: 34668105 Free PMC article.
-
Arabidopsis Zinc-Finger-Like Protein ASYMMETRIC LEAVES2 (AS2) and Two Nucleolar Proteins Maintain Gene Body DNA Methylation in the Leaf Polarity Gene ETTIN (ARF3).Plant Cell Physiol. 2018 Jul 1;59(7):1385-1397. doi: 10.1093/pcp/pcy031. Plant Cell Physiol. 2018. PMID: 29415182
-
The complex of ASYMMETRIC LEAVES (AS) proteins plays a central role in antagonistic interactions of genes for leaf polarity specification in Arabidopsis.Wiley Interdiscip Rev Dev Biol. 2015 Nov-Dec;4(6):655-71. doi: 10.1002/wdev.196. Epub 2015 Jun 24. Wiley Interdiscip Rev Dev Biol. 2015. PMID: 26108442 Free PMC article. Review.
Cited by
-
Arabidopsis ASYMMETRIC LEAVES2 and Nucleolar Factors Are Coordinately Involved in the Perinucleolar Patterning of AS2 Bodies and Leaf Development.Plants (Basel). 2023 Oct 19;12(20):3621. doi: 10.3390/plants12203621. Plants (Basel). 2023. PMID: 37896084 Free PMC article.
-
Arabidopsis histone H3 lysine 9 methyltransferases KYP/SUVH5/6 are involved in leaf development by interacting with AS1-AS2 to repress KNAT1 and KNAT2.Commun Biol. 2023 Feb 24;6(1):219. doi: 10.1038/s42003-023-04607-6. Commun Biol. 2023. PMID: 36828846 Free PMC article.
-
Patterning a Leaf by Establishing Polarities.Front Plant Sci. 2020 Oct 30;11:568730. doi: 10.3389/fpls.2020.568730. eCollection 2020. Front Plant Sci. 2020. PMID: 33193497 Free PMC article. Review.
-
Roles of ASYMMETRIC LEAVES2 (AS2) and Nucleolar Proteins in the Adaxial-Abaxial Polarity Specification at the Perinucleolar Region in Arabidopsis.Int J Mol Sci. 2020 Oct 3;21(19):7314. doi: 10.3390/ijms21197314. Int J Mol Sci. 2020. PMID: 33022996 Free PMC article. Review.
-
Epitope Fine Mapping by Mass Spectrometry: Investigations of Immune Complexes Consisting of Monoclonal Anti-HpTGEKP Antibody and Zinc Finger Protein Linker Phospho-Hexapeptides.Chembiochem. 2022 Oct 19;23(20):e202200390. doi: 10.1002/cbic.202200390. Epub 2022 Sep 12. Chembiochem. 2022. PMID: 35950614 Free PMC article.
References
-
- Bowman, J.L. and Floyd, S.K. (2008) Patterning and polarity in seed plant shoots. Annu. Rev. Plant Biol. 59, 67–88. - PubMed
-
- Byrne, M.E. , Barley, R. , Curtis, M. , Arroyo, J.M. , Dunham, M. , Hudson, A. and Martienssen, R.A. (2000) Asymmetric leaves1 mediates leaf patterning and stem cell function in Arabidopsis . Nature, 408, 967–971. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

