Celastrol inhibits migration, proliferation and transforming growth factor-β2-induced epithelial-mesenchymal transition in lens epithelial cells
- PMID: 31637185
- PMCID: PMC6796093
- DOI: 10.18240/ijo.2019.10.01
Celastrol inhibits migration, proliferation and transforming growth factor-β2-induced epithelial-mesenchymal transition in lens epithelial cells
Abstract
Aim: To investigate the mechanism of celastrol in inhibiting lens epithelial cells (LECs) fibrosis, which is the pathological basis of cataract.
Methods: Human LEC line SRA01/04 was treated with celastrol and transforming growth factor-β2 (TGF-β2). Wound-healing assay, proliferation assay, flow cytometry, real-time polymerase chain reaction (PCR), Western blot and immunocytochemical staining were used to detect the pathological changes of celastrol on LECs. Then, we cultured Sprague-Dawley rat lens in medium as a semi-in vivo model to find the function of celastrol further.
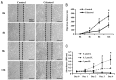
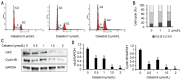
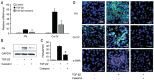
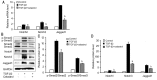
Results: We found that celastrol inhibited the migration of LECs, as well as proliferation (P<0.05). In addition, it induced the G2/M phase arrest by cell cycle-related proteins (P<0.01). Moreover, celastrol inhibited epithelial-mesenchymal transition (EMT) by the blockade of TGF-β/Smad and Jagged/Notch signaling pathways.
Conclusion: Our study demonstrates that celastrol could inhibit TGF-β2-induced lens fibrosis and raises the possibility that celastrol could be a potential novel drug in prevention and treatment of fibrotic cataract.
Keywords: cataract; celastrol; fibrosis; lens; transforming growth factor-β2.
International Journal of Ophthalmology Press.
Figures
Similar articles
-
Arginase-1 promotes lens epithelial-to-mesenchymal transition in different models of anterior subcapsular cataract.Cell Commun Signal. 2023 Sep 18;21(1):236. doi: 10.1186/s12964-023-01210-4. Cell Commun Signal. 2023. PMID: 37723490 Free PMC article.
-
Autophagy inhibition attenuates TGF-β2-induced epithelial-mesenchymal transition in lens epithelial cells.Life Sci. 2021 Jan 15;265:118741. doi: 10.1016/j.lfs.2020.118741. Epub 2020 Nov 10. Life Sci. 2021. PMID: 33181173
-
Bit1-a potential positive regulator of epithelial-mesenchymal transition in lens epithelial cells.Graefes Arch Clin Exp Ophthalmol. 2016 Jul;254(7):1311-8. doi: 10.1007/s00417-016-3357-3. Epub 2016 Apr 28. Graefes Arch Clin Exp Ophthalmol. 2016. PMID: 27122244
-
Blockade of Jagged/Notch pathway abrogates transforming growth factor β2-induced epithelial-mesenchymal transition in human retinal pigment epithelium cells.Curr Mol Med. 2014 May;14(4):523-34. doi: 10.2174/1566524014666140331230411. Curr Mol Med. 2014. PMID: 24694299 Review.
-
Fibrosis in the lens. Sprouty regulation of TGFβ-signaling prevents lens EMT leading to cataract.Exp Eye Res. 2016 Jan;142:92-101. doi: 10.1016/j.exer.2015.02.004. Epub 2015 May 21. Exp Eye Res. 2016. PMID: 26003864 Free PMC article. Review.
Cited by
-
Identification of Putative Non-Substrate-Based XT-I Inhibitors by Natural Product Library Screening.Biomolecules. 2020 Oct 21;10(10):1467. doi: 10.3390/biom10101467. Biomolecules. 2020. PMID: 33096778 Free PMC article.
-
Changes in PEDF, MMP-2, and TGF-β2 levels in the aqueous humor of cataract patients and their correlation with disease severity.Clinics (Sao Paulo). 2024 Oct 15;79:100402. doi: 10.1016/j.clinsp.2024.100402. eCollection 2024. Clinics (Sao Paulo). 2024. PMID: 39413500 Free PMC article.
-
The Promising Therapeutic Potential of Celastrol for Fibrotic Diseases: A Systematic Literature Review on Its Mechanism.Cureus. 2023 Aug 28;15(8):e44269. doi: 10.7759/cureus.44269. eCollection 2023 Aug. Cureus. 2023. PMID: 37772226 Free PMC article. Review.
-
Role and Mechanism of Epithelial-Mesenchymal Transition Mediated by Inflammatory Stress-Induced TGF-β1 in Promoting Arteriovenous Fistula Stenosis.Evid Based Complement Alternat Med. 2022 Jul 6;2022:9454843. doi: 10.1155/2022/9454843. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 37671238 Free PMC article.
-
Celastrol Loaded Nanoparticles With ROS-Response and ROS-Inducer for the Treatment of Ovarian Cancer.Front Chem. 2020 Oct 30;8:574614. doi: 10.3389/fchem.2020.574614. eCollection 2020. Front Chem. 2020. PMID: 33195064 Free PMC article.
References
-
- Dobrzynski JM, Kostis JB. Statins and cataracts: a visual insight. Curr Atheroscler Rep. 2015;17(2):477. - PubMed
-
- Liu YC, Wilkins M, Kim T, Malyugin B, Mehta JS. Cataracts. Lancet. 2017;390(10094):600–612. - PubMed
-
- Awasthi N, Guo SQ, Wagner BJ. Posterior capsular opacification: a problem reduced but not yet eradicated. Arch Ophthalmol. 2009;127(4):555–562. - PubMed
LinkOut - more resources
Full Text Sources
