Calcium Regulates HCC Proliferation as well as EGFR Recycling/Degradation and Could Be a New Therapeutic Target in HCC
- PMID: 31635301
- PMCID: PMC6826902
- DOI: 10.3390/cancers11101588
Calcium Regulates HCC Proliferation as well as EGFR Recycling/Degradation and Could Be a New Therapeutic Target in HCC
Abstract
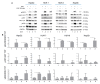
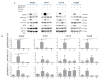
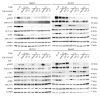
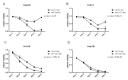
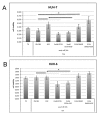
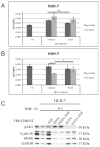
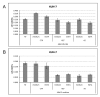
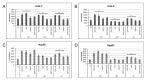
Calcium is the most abundant element in the human body. Its role is essential in physiological and biochemical processes such as signal transduction from outside to inside the cell between the cells of an organ, as well as the release of neurotransmitters from neurons, muscle contraction, fertilization, bone building, and blood clotting. As a result, intra- and extracellular calcium levels are tightly regulated by the body. The liver is the most specialized organ of the body, as its functions, carried out by hepatocytes, are strongly governed by calcium ions. In this work, we analyze the role of calcium in human hepatoma (HCC) cell lines harboring a wild type form of the Epidermal Growth Factor Receptor (EGFR), particularly its role in proliferation and in EGFR downmodulation. Our results highlight that calcium is involved in the proliferative capability of HCC cells, as its subtraction is responsible for EGFR degradation by proteasome machinery and, as a consequence, for EGFR intracellular signaling downregulation. However, calcium-regulated EGFR signaling is cell line-dependent. In cells responding weakly to the epidermal growth factor (EGF), calcium seems to have an opposite effect on EGFR internalization/degradation mechanisms. These results suggest that besides EGFR, calcium could be a new therapeutic target in HCC.
Keywords: AZD9291; BAPTA_AM; EGFR degradation; HCC; calcium ions.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



Similar articles
-
Mathematical model for the effects of epidermal growth factor receptor trafficking dynamics on fibroblast proliferation responses.Biotechnol Prog. 1992 Mar-Apr;8(2):132-43. doi: 10.1021/bp00014a007. Biotechnol Prog. 1992. PMID: 1368006
-
HCRP1 downregulation promotes hepatocellular carcinoma cell migration and invasion through the induction of EGFR activation and epithelial-mesenchymal transition.Biomed Pharmacother. 2017 Apr;88:421-429. doi: 10.1016/j.biopha.2017.01.013. Epub 2017 Jan 22. Biomed Pharmacother. 2017. PMID: 28122307
-
Epidermal growth factor receptor signaling in hepatocellular carcinoma: inflammatory activation and a new intracellular regulatory mechanism.Dig Dis. 2012;30(5):524-31. doi: 10.1159/000341705. Epub 2012 Oct 24. Dig Dis. 2012. PMID: 23108309
-
Reticulocalbin-2 enhances hepatocellular carcinoma proliferation via modulating the EGFR-ERK pathway.Oncogene. 2017 Nov 30;36(48):6691-6700. doi: 10.1038/onc.2017.230. Epub 2017 Jul 24. Oncogene. 2017. PMID: 28745317
-
Impact of IGF-1R/EGFR cross-talks on hepatoma cell sensitivity to gefitinib.Int J Cancer. 2006 Dec 1;119(11):2557-66. doi: 10.1002/ijc.22221. Int J Cancer. 2006. PMID: 16988945
Cited by
-
Hepatocellular Carcinoma: Special Issue Highlights.Cancers (Basel). 2020 Jul 24;12(8):2026. doi: 10.3390/cancers12082026. Cancers (Basel). 2020. PMID: 32722037 Free PMC article.
-
Sorcin promotes migration in cancer and regulates the EGF-dependent EGFR signaling pathways.Cell Mol Life Sci. 2023 Jul 13;80(8):202. doi: 10.1007/s00018-023-04850-4. Cell Mol Life Sci. 2023. PMID: 37442828 Free PMC article.
-
Genetic Polymorphism of Epidermal Growth Factor Gene as a Predictor of Hepatocellular Carcinoma in Hepatitis C Cirrhotic Patients.Asian Pac J Cancer Prev. 2020 Jul 1;21(7):2047-2053. doi: 10.31557/APJCP.2020.21.7.2047. Asian Pac J Cancer Prev. 2020. PMID: 32711431 Free PMC article.
-
Alpinumisoflavone Impairs Mitochondrial Respiration via Oxidative Stress and MAPK/PI3K Regulation in Hepatocellular Carcinoma Cells.Antioxidants (Basel). 2022 Sep 28;11(10):1929. doi: 10.3390/antiox11101929. Antioxidants (Basel). 2022. PMID: 36290652 Free PMC article.
-
PGV-1 Causes Disarrangement of Spindle Microtubule Organization Resulting in Aberrant Mitosis in HLF and HuH6 Cells Associated with Altered MYCN Status.Adv Pharm Bull. 2024 Oct;14(3):665-674. doi: 10.34172/apb.2024.058. Epub 2024 Jul 31. Adv Pharm Bull. 2024. PMID: 39494255 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

