Recent Insights Into Mechanisms of β-Cell Lipo- and Glucolipotoxicity in Type 2 Diabetes
- PMID: 31628942
- PMCID: PMC7073302
- DOI: 10.1016/j.jmb.2019.09.016
Recent Insights Into Mechanisms of β-Cell Lipo- and Glucolipotoxicity in Type 2 Diabetes
Abstract
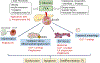
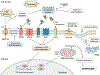
The deleterious effects of chronically elevated free fatty acid (FFA) levels on glucose homeostasis are referred to as lipotoxicity, and the concurrent exposure to high glucose may cause synergistic glucolipotoxicity. Lipo- and glucolipotoxicity have been studied for over 25 years. Here, we review the current evidence supporting the role of pancreatic β-cell lipo- and glucolipotoxicity in type 2 diabetes (T2D), including lipid-based interventions in humans, prospective epidemiological studies, and human genetic findings. In addition to total FFA quantity, the quality of FFAs (saturation and chain length) is a key determinant of lipotoxicity. We discuss in vitro and in vivo experimental models to investigate lipo- and glucolipotoxicity in β-cells and describe experimental pitfalls. Lipo- and glucolipotoxicity adversely affect many steps of the insulin production and secretion process. The molecular mechanisms underpinning lipo- and glucolipotoxic β-cell dysfunction and death comprise endoplasmic reticulum stress, oxidative stress and mitochondrial dysfunction, impaired autophagy, and inflammation. Crosstalk between these stress pathways exists at multiple levels and may aggravate β-cell lipo- and glucolipotoxicity. Lipo- and glucolipotoxicity are therapeutic targets as several drugs impact the underlying stress responses in β-cells, potentially contributing to their glucose-lowering effects in T2D.
Keywords: Insulin; Islet; Lipotoxicity; Palmitate; Pancreatic β-cell.
Crown Copyright © 2019. Published by Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Molecular mechanisms of lipotoxicity-induced pancreatic β-cell dysfunction.Int Rev Cell Mol Biol. 2021;359:357-402. doi: 10.1016/bs.ircmb.2021.02.013. Epub 2021 Mar 12. Int Rev Cell Mol Biol. 2021. PMID: 33832653 Review.
-
Glucolipotoxicity and beta cells in type 2 diabetes mellitus: target for durable therapy?Diabetes Res Clin Pract. 2011 Aug;93 Suppl 1:S37-46. doi: 10.1016/S0168-8227(11)70012-2. Diabetes Res Clin Pract. 2011. PMID: 21864750 Review.
-
The Dynamic Effects of Isosteviol on Insulin Secretion and Its Inability to Counteract the Impaired β-Cell Function during Gluco-, Lipo-, and Aminoacidotoxicity: Studies In Vitro.Nutrients. 2018 Jan 26;10(2):127. doi: 10.3390/nu10020127. Nutrients. 2018. PMID: 29373526 Free PMC article.
-
The Role of CD36 in Type 2 Diabetes Mellitus: β-Cell Dysfunction and Beyond.Diabetes Metab J. 2020 Apr;44(2):222-233. doi: 10.4093/dmj.2020.0053. Diabetes Metab J. 2020. PMID: 32347024 Free PMC article. Review.
-
Metabolic signatures suggest o-phosphocholine to UDP-N-acetylglucosamine ratio as a potential biomarker for high-glucose and/or palmitate exposure in pancreatic β-cells.Metabolomics. 2019 Mar 29;15(4):55. doi: 10.1007/s11306-019-1516-3. Metabolomics. 2019. PMID: 30927092
Cited by
-
Mechanisms of Beta-Cell Apoptosis in Type 2 Diabetes-Prone Situations and Potential Protection by GLP-1-Based Therapies.Int J Mol Sci. 2021 May 18;22(10):5303. doi: 10.3390/ijms22105303. Int J Mol Sci. 2021. PMID: 34069914 Free PMC article. Review.
-
Regulatory roles of CARD9-BCL10-Rac1 (CBR) signalome in islet β-cell function in health and metabolic stress: Is there room for MALT1?Biochem Pharmacol. 2023 Dec;218:115889. doi: 10.1016/j.bcp.2023.115889. Epub 2023 Oct 29. Biochem Pharmacol. 2023. PMID: 37991197 Free PMC article. Review.
-
Botanical Interventions to Improve Glucose Control and Options for Diabetes Therapy.SN Compr Clin Med. 2021 Dec;3(12):2465-2491. doi: 10.1007/s42399-021-01034-8. Epub 2021 Aug 15. SN Compr Clin Med. 2021. PMID: 35098034 Free PMC article.
-
Very-Long-Chain Unsaturated Sphingolipids Mediate Oleate-Induced Rat β-Cell Proliferation.Diabetes. 2022 Jun 1;71(6):1218-1232. doi: 10.2337/db21-0640. Diabetes. 2022. PMID: 35287172 Free PMC article.
-
Molecular and Cellular Bases of Lipodystrophy Syndromes.Front Endocrinol (Lausanne). 2022 Jan 3;12:803189. doi: 10.3389/fendo.2021.803189. eCollection 2021. Front Endocrinol (Lausanne). 2022. PMID: 35046902 Free PMC article. Review.
References
-
- Organization WH. Obesity and overweight. 2018; February 16.
-
- Lewis GF, Carpentier A, Adeli K, Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev. 2002;23:201–29. - PubMed
-
- Prentki M, Corkey BE. Are the beta-cell signaling molecules malonyl-CoA and cystolic long-chain acyl-CoA implicated in multiple tissue defects of obesity and NIDDM? Diabetes. 1996;45:273–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

