Cytomegalovirus Seropositivity Is Associated With Increased Microbial Translocation in People Living With Human Immunodeficiency Virus and Uninfected Controls
- PMID: 31608409
- PMCID: PMC7486843
- DOI: 10.1093/cid/ciz1001
Cytomegalovirus Seropositivity Is Associated With Increased Microbial Translocation in People Living With Human Immunodeficiency Virus and Uninfected Controls
Abstract
Background: Cytomegalovirus (CMV) seropositivity and anti-CMV immunoglobulin G (IgG) levels are associated with adverse health outcomes in elderly populations. Among people living with human immunodeficiency virus (PLWH), CMV seropositivity has been associated with persistent CD8 T-cell elevation and increased risk of developing non-AIDS comorbidities despite long-term antiretroviral therapy (ART). Herein, we investigated whether CMV seropositivity and elevation of anti-CMV IgG levels were associated with increased epithelial gut damage, microbial translocation, and systemic inflammation.
Methods: A total of 150 PLWH (79 ART-naive and 71 ART-treated) were compared to 26 without human immunodeficiency virus (HIV) infection (uninfected controls). Plasma markers of HIV disease progression, epithelial gut damage, microbial translocation, nonspecific B-cell activation, anti-CMV and anti-Epstein-Barr virus (EBV) IgG levels, and proinflammatory cytokines were measured.
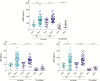
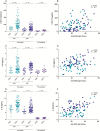
Results: CMV seropositivity and elevated anti-CMV IgG levels were associated with markers of epithelial gut damage, microbial translocation, and inflammation in PLWH and participants without HIV infection. In contrast, total nonspecific IgG, immunoglobulin M, immunoglobulin A, and anti-EBV IgG levels were not associated with these markers. CMV seropositivity was associated with markers of epithelial gut damage, microbial translocation, and inflammation independent of sociodemographic and behavioral characteristics of the study population.
Conclusions: CMV-seropositive people with and without HIV had increased epithelial gut damage, microbial translocation, and inflammation. Furthermore, anti-CMV IgG levels were independently associated with increased epithelial gut damage and microbial translocation. CMV coinfection may partially explain persistent gut damage, microbial translocation, and inflammation in ART-treated PLWH.
Keywords: HIV; cytomegalovirus; epithelial gut damage; inflammation; microbial translocation.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures

Similar articles
-
Cytomegalovirus as an Uninvited Guest in the Response to Vaccines in People Living with HIV.Viruses. 2021 Jun 29;13(7):1266. doi: 10.3390/v13071266. Viruses. 2021. PMID: 34209711 Free PMC article. Review.
-
Influence of letermovir treatment on gut inflammation in people living with HIV on antiretroviral therapy: protocol of the open-label controlled randomised CIAO study.BMJ Open. 2023 Jan 23;13(1):e067640. doi: 10.1136/bmjopen-2022-067640. BMJ Open. 2023. PMID: 36690406 Free PMC article.
-
Subclinical Cytomegalovirus DNA Is Associated with CD4 T Cell Activation and Impaired CD8 T Cell CD107a Expression in People Living with HIV despite Early Antiretroviral Therapy.J Virol. 2019 Jun 14;93(13):e00179-19. doi: 10.1128/JVI.00179-19. Print 2019 Jul 1. J Virol. 2019. PMID: 31019052 Free PMC article.
-
Asymptomatic CMV Replication During Early Human Immunodeficiency Virus (HIV) Infection Is Associated With Lower CD4/CD8 Ratio During HIV Treatment.Clin Infect Dis. 2016 Dec 1;63(11):1517-1524. doi: 10.1093/cid/ciw612. Epub 2016 Sep 6. Clin Infect Dis. 2016. PMID: 27601222 Free PMC article.
-
The Bacterium Akkermansia muciniphila: A Sentinel for Gut Permeability and Its Relevance to HIV-Related Inflammation.Front Immunol. 2020 Apr 9;11:645. doi: 10.3389/fimmu.2020.00645. eCollection 2020. Front Immunol. 2020. PMID: 32328074 Free PMC article. Review.
Cited by
-
A Summary of the Fifth Annual Virology Education HIV Microbiome Workshop.AIDS Res Hum Retroviruses. 2020 Nov;36(11):886-895. doi: 10.1089/AID.2020.0121. Epub 2020 Sep 7. AIDS Res Hum Retroviruses. 2020. PMID: 32777940 Free PMC article.
-
Cytomegalovirus as an Uninvited Guest in the Response to Vaccines in People Living with HIV.Viruses. 2021 Jun 29;13(7):1266. doi: 10.3390/v13071266. Viruses. 2021. PMID: 34209711 Free PMC article. Review.
-
Chronic obstructive pulmonary disease in HIV.Expert Rev Respir Med. 2021 Jan;15(1):71-87. doi: 10.1080/17476348.2021.1848556. Epub 2020 Nov 23. Expert Rev Respir Med. 2021. PMID: 33167728 Free PMC article. Review.
-
Plasma 1,3-β-d-glucan levels predict adverse clinical outcomes in critical illness.JCI Insight. 2021 Jul 22;6(14):e141277. doi: 10.1172/jci.insight.141277. JCI Insight. 2021. PMID: 34128840 Free PMC article.
-
Immunological age prediction in HIV-infected, ART-treated individuals.Aging (Albany NY). 2021 Oct 11;13(19):22772-22791. doi: 10.18632/aging.203625. Epub 2021 Oct 11. Aging (Albany NY). 2021. PMID: 34635604 Free PMC article.
References
-
- Klenerman P, Oxenius A. T cell responses to cytomegalovirus. Nat Rev Immunol 2016; 16:367–77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

