Differential Susceptibility and Innate Immune Response of Aedes aegypti and Aedes albopictus to the Haitian Strain of the Mayaro Virus
- PMID: 31601017
- PMCID: PMC6832402
- DOI: 10.3390/v11100924
Differential Susceptibility and Innate Immune Response of Aedes aegypti and Aedes albopictus to the Haitian Strain of the Mayaro Virus
Abstract
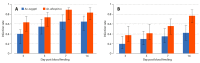
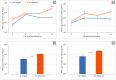
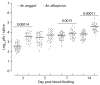
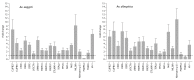
Mayaro (MAYV) is an emerging arthropod-borne virus belonging to the Alphavirus genus of the Togaviridae family. Although forest-dwelling Haemagogus mosquitoes have been considered as its main vector, the virus has also been detected in circulating Aedes ssp mosquitoes. Here we assess the susceptibility of Aedes aegypti and Aedes albopictus to infection with MAYV and their innate immune response at an early stage of infection. Aedes albopictus was more susceptible to infection with MAYV than Ae. aegypti. Analysis of transcript levels of twenty immunity-related genes by real-time PCR in the midgut of both mosquitoes infected with MAYV revealed increased expression of several immune genes, including CLIP-domain serine proteases, the anti-microbial peptides defensin A, E, cecropin E, and the virus inducible gene. The regulation of certain genes appeared to be Aedes species-dependent. Infection of Ae. aegypti with MAYV resulted in increased levels of myeloid differentiation2-related lipid recognition protein (ML26A) transcripts, as compared to Ae. albopictus. Increased expression levels of thio-ester-containing protein 22 (TEP22) and Niemann-Pick type C1 (NPC1) gene transcripts were observed in infected Ae. albopictus, but not Ae. aegypti. The differences in these gene expression levels during MAYV infection could explain the variation in susceptibility observed in both mosquito species.
Keywords: Aedes aegypti; Aedes albopictus; ML26A; Mayaro virus; Niemann–Pick type C1; arbovirus; chondrocytes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Transmission potential of Mayaro virus in Florida Aedes aegypti and Aedes albopictus mosquitoes.Med Vet Entomol. 2018 Dec;32(4):436-442. doi: 10.1111/mve.12322. Epub 2018 Jul 13. Med Vet Entomol. 2018. PMID: 30006976
-
Differential Susceptibility of Aedes aegypti and Aedes albopictus Mosquitoes to Infection by Mayaro Virus Strains.Am J Trop Med Hyg. 2023 May 30;109(1):115-122. doi: 10.4269/ajtmh.22-0777. Print 2023 Jul 5. Am J Trop Med Hyg. 2023. PMID: 37253447 Free PMC article.
-
Vector competence of Aedes aegypti, Aedes albopictus, and Culex quinquefasciatus mosquitoes for Mayaro virus.PLoS Negl Trop Dis. 2020 Apr 14;14(4):e0007518. doi: 10.1371/journal.pntd.0007518. eCollection 2020 Apr. PLoS Negl Trop Dis. 2020. PMID: 32287269 Free PMC article.
-
GloPID-R report on chikungunya, o'nyong-nyong and Mayaro virus, part 5: Entomological aspects.Antiviral Res. 2020 Feb;174:104670. doi: 10.1016/j.antiviral.2019.104670. Epub 2019 Dec 5. Antiviral Res. 2020. PMID: 31812638 Review.
-
[Mayaro: a re-emerging Arbovirus in Venezuela and Latin America].Biomedica. 2012 Jun;32(2):286-302. doi: 10.1590/S0120-41572012000300017. Biomedica. 2012. PMID: 23242303 Review. Spanish.
Cited by
-
Wolbachia wPip Blocks Zika Virus Transovarial Transmission in Aedes albopictus.Microbiol Spectr. 2022 Oct 26;10(5):e0263321. doi: 10.1128/spectrum.02633-21. Epub 2022 Jul 27. Microbiol Spectr. 2022. PMID: 35894613 Free PMC article.
-
Adenoviral-Vectored Mayaro and Chikungunya Virus Vaccine Candidates Afford Partial Cross-Protection From Lethal Challenge in A129 Mouse Model.Front Immunol. 2020 Nov 4;11:591885. doi: 10.3389/fimmu.2020.591885. eCollection 2020. Front Immunol. 2020. PMID: 33224148 Free PMC article.
-
Epidemic Alphaviruses: Ecology, Emergence and Outbreaks.Microorganisms. 2020 Aug 1;8(8):1167. doi: 10.3390/microorganisms8081167. Microorganisms. 2020. PMID: 32752150 Free PMC article. Review.
-
Favipiravir Inhibits Mayaro Virus Infection in Mice.Viruses. 2021 Nov 3;13(11):2213. doi: 10.3390/v13112213. Viruses. 2021. PMID: 34835018 Free PMC article.
-
Coupled small molecules target RNA interference and JAK/STAT signaling to reduce Zika virus infection in Aedes aegypti.PLoS Pathog. 2022 Apr 4;18(4):e1010411. doi: 10.1371/journal.ppat.1010411. eCollection 2022 Apr. PLoS Pathog. 2022. PMID: 35377915 Free PMC article.
References
-
- Phillips P., Lehmann D., Spooner V., Barker J., Tulloch S., Sungu M., Canil K., Pratt R., Lupiwa T., Alpers M. Viruses associated with acute lower respiratory tract infections in children from the eastern highlands of Papua New Guinea (1983–1985) Southeast. Asian J. Trop. Med. Public Health. 1990;21:373–382. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

