Acteoside inhibits inflammatory response via JAK/STAT signaling pathway in osteoarthritic rats
- PMID: 31590658
- PMCID: PMC6781407
- DOI: 10.1186/s12906-019-2673-7
Acteoside inhibits inflammatory response via JAK/STAT signaling pathway in osteoarthritic rats
Abstract
Background: Osteoarthritis (OA) is a common degenerative disease of synovial joints caused by inflammation. Acteoside (ACT), a major component and lipase inhibitor from the Chinese tea Ligustrum purpurascens kudingcha, has been reported to regulate the inflammation and immune response. The study aims to investigate the effects of ACT on inflammatory responses and joint protection in OA rats.
Methods: Cell proliferation was examined by MTT and colony formation assay. Apoptosis was analyzed using flow cytometry with Annexin V/PI staining. ELISA was employed to examine the concentration of inflammatory cytokines. OA rat model was established by surgery stimulation.
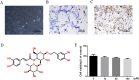
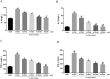
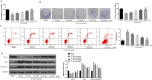
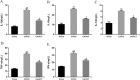
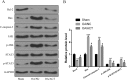
Results: ACT treatment significantly inhibited the upregulation of inflammatory cytokines induced by IL-1β in primary chondrocytes, including IL-6, IL-12, TNF-α and IFN-γ. ACT stimulation also enhanced the cell proliferation, while inhibited cell apoptosis in IL-1β-treated chondrocytes. Consistently, ACT treatment led to downregulation of cleaved-caspase-3 and apoptosis regulator Bax, and upregulation of Bcl-2. Furthermore, ACT treatment inhibited IL-1β-induced activation of JAK/STAT pathway. The results were confirmed in surgery-induced OA rat model. Moreover, ACT treatment significantly inhibited synovial inflammation and articular chondrocyte apoptosis in OA rats.
Conclusion: Our findings indicate that ACT has the potential therapeutic effect on OA through inhibiting the inflammatory responses via inactivating JAK/STAT signaling pathway.
Keywords: Acteoside; Apoptosis; Inflammation; JAK/STAT; Osteoarthritis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Downregulation of long noncoding RNA LOC101928134 inhibits the synovial hyperplasia and cartilage destruction of osteoarthritis rats through the activation of the Janus kinase/signal transducers and activators of transcription signaling pathway by upregulating IFNA1.J Cell Physiol. 2019 Jul;234(7):10523-10534. doi: 10.1002/jcp.27730. Epub 2018 Nov 19. J Cell Physiol. 2019. PMID: 30456844
-
[Mechanism of Huangqin Qingre Chubi Capsules regulating p53/p21 signaling pathway to delay chondrocyte senescence in osteoarthritic rats].Zhongguo Zhong Yao Za Zhi. 2024 Jun;49(12):3330-3339. doi: 10.19540/j.cnki.cjcmm.20240318.702. Zhongguo Zhong Yao Za Zhi. 2024. PMID: 39041096 Chinese.
-
The elevated expression of IL-38 serves as an anti-inflammatory factor in osteoarthritis and its protective effect in osteoarthritic chondrocytes.Int Immunopharmacol. 2021 May;94:107489. doi: 10.1016/j.intimp.2021.107489. Epub 2021 Mar 25. Int Immunopharmacol. 2021. PMID: 33774357
-
The role of cytokines in osteoarthritis pathophysiology.Biorheology. 2002;39(1-2):237-46. Biorheology. 2002. PMID: 12082286 Review.
-
The potential roles of JAK/STAT signaling in the progression of osteoarthritis.Front Endocrinol (Lausanne). 2022 Nov 24;13:1069057. doi: 10.3389/fendo.2022.1069057. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36506076 Free PMC article. Review.
Cited by
-
Acteoside Counteracts Interleukin-1β-Induced Catabolic Processes through the Modulation of Mitogen-Activated Protein Kinases and the NFκB Cellular Signaling Pathway.Oxid Med Cell Longev. 2021 Mar 24;2021:8684725. doi: 10.1155/2021/8684725. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 33833854 Free PMC article.
-
In vitro characterization of iridoid and phenylethanoid glycosides from Cistanche phelypaea for nutraceutical and pharmacological applications.Phytother Res. 2022 Nov;36(11):4155-4166. doi: 10.1002/ptr.7548. Epub 2022 Jul 3. Phytother Res. 2022. PMID: 35781895 Free PMC article.
-
FOXM1 activates JAK1/STAT3 pathway in human osteoarthritis cartilage cell inflammatory reaction.Exp Biol Med (Maywood). 2021 Mar;246(6):644-653. doi: 10.1177/1535370220974933. Epub 2020 Dec 9. Exp Biol Med (Maywood). 2021. PMID: 33297736 Free PMC article.
-
Perturbations in Neuroinflammatory Pathways Are Associated With a Worst Pain Profile in Oncology Patients Receiving Chemotherapy.J Pain. 2023 Jan;24(1):84-97. doi: 10.1016/j.jpain.2022.08.007. Epub 2022 Sep 15. J Pain. 2023. PMID: 36115520 Free PMC article.
-
Parathyroid hormone (1-34) ameliorates cartilage degeneration and subchondral bone deterioration in collagenase-induced osteoarthritis model in mice.Bone Joint Res. 2020 Oct 16;9(10):675-688. doi: 10.1302/2046-3758.910.BJR-2020-0018.R1. eCollection 2020 Oct. Bone Joint Res. 2020. PMID: 33101657 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

