SV40 Hijacks Cellular Transport, Membrane Penetration, and Disassembly Machineries to Promote Infection
- PMID: 31590347
- PMCID: PMC6832212
- DOI: 10.3390/v11100917
SV40 Hijacks Cellular Transport, Membrane Penetration, and Disassembly Machineries to Promote Infection
Abstract
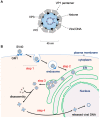
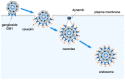
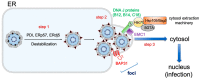
During entry, a virus must be transported through the endomembrane system of the host cell, penetrate a cellular membrane, and undergo capsid disassembly, to reach the cytosol and often the nucleus in order to cause infection. To do so requires the virus to coordinately exploit the action of cellular membrane transport, penetration, and disassembly machineries. How this is accomplished remains enigmatic for many viruses, especially for viruses belonging to the nonenveloped virus family. In this review, we present the current model describing infectious entry of the nonenveloped polyomavirus (PyV) SV40. Insights from SV40 entry are likely to provide strategies to combat PyV-induced diseases, and to illuminate cellular trafficking, membrane transport, and disassembly mechanisms.
Keywords: SV40; endoplasmic reticulum; membrane penetration; nonenveloped virus; viral disassembly.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results
Figures
Similar articles
-
A nucleotide exchange factor promotes endoplasmic reticulum-to-cytosol membrane penetration of the nonenveloped virus simian virus 40.J Virol. 2015 Apr;89(8):4069-79. doi: 10.1128/JVI.03552-14. Epub 2015 Feb 4. J Virol. 2015. PMID: 25653441 Free PMC article.
-
The endoplasmic reticulum membrane J protein C18 executes a distinct role in promoting simian virus 40 membrane penetration.J Virol. 2015 Apr;89(8):4058-68. doi: 10.1128/JVI.03574-14. Epub 2015 Jan 28. J Virol. 2015. PMID: 25631089 Free PMC article.
-
Bag2 Is a Component of a Cytosolic Extraction Machinery That Promotes Membrane Penetration of a Nonenveloped Virus.J Virol. 2018 Jul 17;92(15):e00607-18. doi: 10.1128/JVI.00607-18. Print 2018 Aug 1. J Virol. 2018. PMID: 29769335 Free PMC article.
-
How Polyomaviruses Exploit the ERAD Machinery to Cause Infection.Viruses. 2016 Aug 29;8(9):242. doi: 10.3390/v8090242. Viruses. 2016. PMID: 27589785 Free PMC article. Review.
-
Breach: Host Membrane Penetration and Entry by Nonenveloped Viruses.Trends Microbiol. 2018 Jun;26(6):525-537. doi: 10.1016/j.tim.2017.09.010. Epub 2017 Oct 25. Trends Microbiol. 2018. PMID: 29079499 Review.
Cited by
-
Evidence of the Mechanism by Which Polyomaviruses Exploit the Extracellular Vesicle Delivery System during Infection.Viruses. 2020 May 27;12(6):585. doi: 10.3390/v12060585. Viruses. 2020. PMID: 32471033 Free PMC article. Review.
-
Golgi-associated BICD adaptors couple ER membrane penetration and disassembly of a viral cargo.J Cell Biol. 2020 May 4;219(5):e201908099. doi: 10.1083/jcb.201908099. J Cell Biol. 2020. PMID: 32259203 Free PMC article.
-
ER functions are exploited by viruses to support distinct stages of their life cycle.Biochem Soc Trans. 2020 Oct 30;48(5):2173-2184. doi: 10.1042/BST20200395. Biochem Soc Trans. 2020. PMID: 33119046 Free PMC article. Review.
-
Mechanisms of substrate processing during ER-associated protein degradation.Nat Rev Mol Cell Biol. 2023 Nov;24(11):777-796. doi: 10.1038/s41580-023-00633-8. Epub 2023 Aug 1. Nat Rev Mol Cell Biol. 2023. PMID: 37528230 Review.
-
Membrane Rafts: Portals for Viral Entry.Front Microbiol. 2021 Feb 4;12:631274. doi: 10.3389/fmicb.2021.631274. eCollection 2021. Front Microbiol. 2021. PMID: 33613502 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

