The splicing FK506-binding protein-51 isoform plays a role in glioblastoma resistance through programmed cell death ligand-1 expression regulation
- PMID: 31583120
- PMCID: PMC6760221
- DOI: 10.1038/s41420-019-0216-0
The splicing FK506-binding protein-51 isoform plays a role in glioblastoma resistance through programmed cell death ligand-1 expression regulation
Abstract
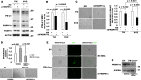
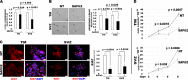
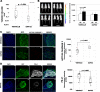
Gliomas aberrantly express programmed cell death ligand-1 (PD-L1), which has a pivotal role in immunoevasion. The splicing isoform of FKBP5, termed FKBP51s, is a PD-L1 foldase, assisting the immune checkpoint molecule in maturation and expression on the plasma membrane. The concept that PD-L1 supports tumor-intrinsic properties is increasingly emerging. The aim of the present work was to confirm the pro-tumoral effect of PD-L1 on human glioma cell survival, stemness capacity and resistance, and to address the issue of whether, by targeting its foldase either chemically or by silencing, the aggressive tumor features could be attenuated. PD-L1-depleted glioma cells have a reduced threshold for apoptosis, while PD-L1 forced expression increases resistance. Similar results were obtained with FKBP51s modulation. The ability of PD-L1 to counteract cell death was hampered by FKBP51s silencing. PD-L1 expression was particularly high in glioma cells with a cancer-stem-cell profile. Moreover, PD-L1 sustained the spheroid formation capability of glioma cells. Targeting of FKBP51s by small-interfering RNA (siRNA) or the specific inhibitor SAFit2, reduced the number of formed spheroids, along with PD-L1 expression. Finally, in an orthotopic mouse model of glioblastoma, daily treatment with SAFit2 significantly reduced tumor PD-L1 expression, and tumor growth. In treated mice, caspase-3 activation and reduced vimentin expression were observed in excised tumors. In conclusion, targeting of FKBP51s hampers PD-L1 and its pro-tumoral properties, thereby affecting the self-renewal and growth capacities of glioblastoma cells in vitro and in vivo.
Keywords: Apoptosis; CNS cancer.
© The Author(s) 2019.
Conflict of interest statement
Conflict of interestThe authors declare that they have no conflict of interest.
Figures



Similar articles
-
A regulatory role for the co-chaperone FKBP51s in PD-L1 expression in glioma.Oncotarget. 2017 Jul 17;8(40):68291-68304. doi: 10.18632/oncotarget.19309. eCollection 2017 Sep 15. Oncotarget. 2017. PMID: 28978117 Free PMC article.
-
PD-L1 Expression Fluctuates Concurrently with Cyclin D in Glioblastoma Cells.Cells. 2021 Sep 9;10(9):2366. doi: 10.3390/cells10092366. Cells. 2021. PMID: 34572014 Free PMC article.
-
Disruption of SIRT7 Increases the Efficacy of Checkpoint Inhibitor via MEF2D Regulation of Programmed Cell Death 1 Ligand 1 in Hepatocellular Carcinoma Cells.Gastroenterology. 2020 Feb;158(3):664-678.e24. doi: 10.1053/j.gastro.2019.10.025. Epub 2019 Oct 31. Gastroenterology. 2020. PMID: 31678303
-
The Prognostic and Therapeutic Value of PD-L1 in Glioma.Front Pharmacol. 2019 Jan 9;9:1503. doi: 10.3389/fphar.2018.01503. eCollection 2018. Front Pharmacol. 2019. PMID: 30687086 Free PMC article. Review.
-
Clinical Neuropathology mini-review 6-2015: PD-L1: emerging biomarker in glioblastoma?Clin Neuropathol. 2015 Nov-Dec;34(6):313-21. doi: 10.5414/np300922. Clin Neuropathol. 2015. PMID: 26501438 Free PMC article. Review.
Cited by
-
The FKBP51s Splice Isoform Predicts Unfavorable Prognosis in Patients with Glioblastoma.Cancer Res Commun. 2024 May 16;4(5):1296-1306. doi: 10.1158/2767-9764.CRC-24-0083. Cancer Res Commun. 2024. PMID: 38651817 Free PMC article.
-
Analysis of the Selective Antagonist SAFit2 as a Chemical Probe for the FK506-Binding Protein 51.ACS Pharmacol Transl Sci. 2023 Feb 14;6(3):361-371. doi: 10.1021/acsptsci.2c00234. eCollection 2023 Mar 10. ACS Pharmacol Transl Sci. 2023. PMID: 36926456 Free PMC article. Review.
-
Detection of isoforms and genomic alterations by high-throughput full-length single-cell RNA sequencing in ovarian cancer.Nat Commun. 2023 Nov 27;14(1):7780. doi: 10.1038/s41467-023-43387-9. Nat Commun. 2023. PMID: 38012143 Free PMC article.
-
Eradication of CSCs: the roadmap for curing cancer.Oncoscience. 2020 Sep 9;7(9-10):70-72. doi: 10.18632/oncoscience.516. eCollection 2020 Sep. Oncoscience. 2020. PMID: 33195737 Free PMC article. No abstract available.
-
Knocking out Fkbp51 decreases CCl4-induced liver injury through enhancement of mitochondrial function and Parkin activity.Cell Biosci. 2024 Jan 2;14(1):1. doi: 10.1186/s13578-023-01184-3. Cell Biosci. 2024. PMID: 38167156 Free PMC article.
References
-
- D’Arrigo, P. et al. Manipulation of the immune system for cancer defeat: a focus on the T cell inhibitory checkpoint molecules. Curr. Med. Chem. (2018). https://doi.org/0.2174/0929867325666181106114421, (Epub ahead of print). - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

