Pseudomonas aeruginosa Quorum Sensing Molecule Alters Skeletal Muscle Protein Homeostasis by Perturbing the Antioxidant Defense System
- PMID: 31575771
- PMCID: PMC6775459
- DOI: 10.1128/mBio.02211-19
Pseudomonas aeruginosa Quorum Sensing Molecule Alters Skeletal Muscle Protein Homeostasis by Perturbing the Antioxidant Defense System
Erratum in
-
Erratum for Bandyopadhaya et al., "Pseudomonas aeruginosa Quorum Sensing Molecule Alters Skeletal Muscle Protein Homeostasis by Perturbing the Antioxidant Defense System".mBio. 2020 Feb 4;11(1):e03316-19. doi: 10.1128/mBio.03316-19. mBio. 2020. PMID: 32019795 Free PMC article. No abstract available.
Abstract
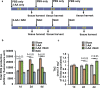
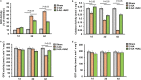
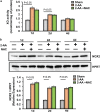
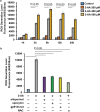
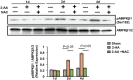
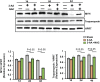
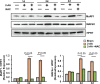
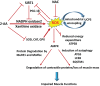
Skeletal muscle function is compromised in many illnesses, including chronic infections. The Pseudomonas aeruginosa quorum sensing (QS) signal, 2-amino acetophenone (2-AA), is produced during acute and chronic infections and excreted in human tissues, including the lungs of cystic fibrosis patients. We have shown that 2-AA facilitates pathogen persistence, likely via its ability to promote the formation of bacterial persister cells, and that it acts as an interkingdom immunomodulatory signal that epigenetically reprograms innate immune functions. Moreover, 2-AA compromises muscle contractility and impacts the expression of genes involved in reactive oxygen species (ROS) homeostasis in skeletal muscle and in mitochondrial functions. Here, we elucidate the molecular mechanisms of 2-AA's impairment of skeletal muscle function and ROS homeostasis. Murine in vivo and differentiated C2C12 myotube cell studies showed that 2-AA promotes ROS generation in skeletal muscle via the modulation of xanthine oxidase (XO) activity, NAD(P)H oxidase2 (NOX2) protein level, and the activity of antioxidant enzymes. ROS accumulation triggers the activity of AMP-activated protein kinase (AMPK), likely upstream of the observed locations of induction of ubiquitin ligases Muscle RING Finger 1 (MuRF1) and Muscle Atrophy F-box (MAFbx), and induces autophagy-related proteins. The protein-level perturbation in skeletal muscle of silent mating type information regulation 2 homolog 1 (SIRT1), peroxisome proliferator-activated receptor gamma coactivator 1 (PGC-1), and uncoupling protein 3 (UCP3) is rescued by the antioxidant N-acetyl-l-cysteine (NAC). Together, these results unveil a novel form of action of a QS bacterial molecule and provide molecular insights into the 2-AA-mediated skeletal muscle dysfunction caused by P. aeruginosaIMPORTANCEPseudomonas aeruginosa, a bacterium that is resistant to treatment, causes serious acute, persistent, and relapsing infections in humans. There is increasing evidence that bacterial excreted small molecules play a critical role during infection. We have shown that a quorum sensing (QS)-regulated excreted small molecule, 2-AA, which is abundantly produced by P. aeruginosa, promotes persistent infections, dampens host inflammation, and triggers mitochondrial dysfunction in skeletal muscle. QS is a cell-to-cell communication system utilized by bacteria to promote collective behaviors. The significance of our study in identifying a mechanism that leads to skeletal muscle dysfunction, via the action of a QS molecule, is that it may open new avenues in the control of muscle loss as a result of infection and sepsis. Given that QS is a common characteristic of prokaryotes, it is possible that 2-AA-like molecules promoting similar effects may exist in other pathogens.
Keywords: 2-amino acetophenone (2-AA); NAD(P)H oxidase 2 (NOX2); Pseudomonas aeruginosa; chronic infections; muscle atrophy; muscle dysfunction; quorum sensing (QS); reactive oxygen species (ROS); skeletal muscle; virulence; xanthine oxidase (XO).
Copyright © 2019 Bandyopadhaya et al.
Figures



Similar articles
-
Skeletal muscle mitochondrial dysfunction mediated by Pseudomonas aeruginosa quorum-sensing transcription factor MvfR: reversing effects with anti-MvfR and mitochondrial-targeted compounds.mBio. 2024 Jul 17;15(7):e0129224. doi: 10.1128/mbio.01292-24. Epub 2024 Jun 11. mBio. 2024. PMID: 38860823 Free PMC article.
-
A small volatile bacterial molecule triggers mitochondrial dysfunction in murine skeletal muscle.PLoS One. 2013 Sep 30;8(9):e74528. doi: 10.1371/journal.pone.0074528. eCollection 2013. PLoS One. 2013. PMID: 24098655 Free PMC article.
-
Bacterial-excreted small volatile molecule 2-aminoacetophenone induces oxidative stress and apoptosis in murine skeletal muscle.Int J Mol Med. 2016 Apr;37(4):867-78. doi: 10.3892/ijmm.2016.2487. Epub 2016 Feb 12. Int J Mol Med. 2016. PMID: 26935176 Free PMC article.
-
The role of quorum sensing in the pathogenicity of the cunning aggressor Pseudomonas aeruginosa.Anal Bioanal Chem. 2007 Jan;387(2):409-14. doi: 10.1007/s00216-006-0774-x. Epub 2006 Sep 26. Anal Bioanal Chem. 2007. PMID: 17019573 Review.
-
Bacteria-Host Crosstalk: Sensing of the Quorum in the Context of Pseudomonas aeruginosa Infections.J Innate Immun. 2019;11(3):263-279. doi: 10.1159/000494069. Epub 2018 Nov 14. J Innate Immun. 2019. PMID: 30428481 Free PMC article. Review.
Cited by
-
Hypoxia enhances autophagy level of human sperms.Sci Rep. 2024 Apr 11;14(1):8465. doi: 10.1038/s41598-024-59213-1. Sci Rep. 2024. PMID: 38605082 Free PMC article.
-
Impact of quorum sensing signaling molecules in gram-negative bacteria on host cells: current understanding and future perspectives.Gut Microbes. 2022 Jan-Dec;14(1):2039048. doi: 10.1080/19490976.2022.2039048. Gut Microbes. 2022. PMID: 35188058 Free PMC article. Review.
-
Mechanisms of the Quorum Sensing Systems of Pseudomonas aeruginosa: Host and Bacteria.Curr Med Chem. 2024;31(35):5755-5767. doi: 10.2174/0929867331666230821110440. Curr Med Chem. 2024. PMID: 37605403 Review.
-
Melanin precursors mediated adaption to temperature changes in fungus and animal via inhibition of lipid-mediated ferroptosis.Sci China Life Sci. 2023 Aug;66(8):1800-1817. doi: 10.1007/s11427-022-2265-6. Epub 2023 Mar 17. Sci China Life Sci. 2023. PMID: 36949229
-
Tryptophan-centered metabolic alterations coincides with lipid-mediated fungal response to cold stress.Heliyon. 2023 Jan 21;9(2):e13066. doi: 10.1016/j.heliyon.2023.e13066. eCollection 2023 Feb. Heliyon. 2023. PMID: 36747564 Free PMC article.
References
-
- Rocheteau P, Chatre L, Briand D, Mebarki M, Jouvion G, Bardon J, Crochemore C, Serrani P, Lecci PP, Latil M, Matot B, Carlier PG, Latronico N, Huchet C, Lafoux A, Sharshar T, Ricchetti M, Chretien F. 2015. Sepsis induces long-term metabolic and mitochondrial muscle stem cell dysfunction amenable by mesenchymal stem cell therapy. Nat Commun 6:10145. doi:10.1038/ncomms10145. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
