Recent Insights on Inflammasomes, Gasdermin Pores, and Pyroptosis
- PMID: 31570336
- PMCID: PMC7197430
- DOI: 10.1101/cshperspect.a036392
Recent Insights on Inflammasomes, Gasdermin Pores, and Pyroptosis
Abstract
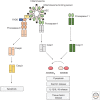
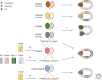
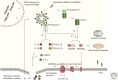
Inflammasomes assemble in the cytosol of myeloid and epithelial cells on sensing of cellular stress and pathogen-associated molecular patterns and serve as scaffolds for recruitment and activation of inflammatory caspases. Inflammasomes play beneficial roles in host and immune responses against diverse pathogens but may also promote inflammatory tissue damage if uncontrolled. Gasdermin D (GSDMD) is a recently identified substrate of murine caspase-1 and caspase-11, and human caspases-1, -4, and -5 that mediates a regulated lytic cell death mode termed pyroptosis. Recent studies have identified pyroptosis as a critical inflammasome effector mechanism that controls inflammasome-dependent cytokine secretion and contributes to antimicrobial defense and inflammasome-mediated autoinflammatory diseases. Here, we review recent developments on inflammasome-associated effector functions with an emphasis on the emerging roles of gasdermin pores and pyroptosis.
Copyright © 2020 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
Similar articles
-
FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation.Nat Immunol. 2020 Jul;21(7):736-745. doi: 10.1038/s41590-020-0669-6. Epub 2020 May 4. Nat Immunol. 2020. PMID: 32367036 Free PMC article.
-
Live-cell visualization of gasdermin D-driven pyroptotic cell death.J Biol Chem. 2017 Sep 1;292(35):14649-14658. doi: 10.1074/jbc.M117.797217. Epub 2017 Jul 18. J Biol Chem. 2017. PMID: 28726636 Free PMC article.
-
Uncoupled pyroptosis and IL-1β secretion downstream of inflammasome signaling.Front Immunol. 2023 Apr 6;14:1128358. doi: 10.3389/fimmu.2023.1128358. eCollection 2023. Front Immunol. 2023. PMID: 37090724 Free PMC article. Review.
-
Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death.Nature. 2015 Oct 29;526(7575):660-5. doi: 10.1038/nature15514. Epub 2015 Sep 16. Nature. 2015. PMID: 26375003
-
Mechanism and Regulation of Gasdermin-Mediated Cell Death.Cold Spring Harb Perspect Biol. 2020 Mar 2;12(3):a036400. doi: 10.1101/cshperspect.a036400. Cold Spring Harb Perspect Biol. 2020. PMID: 31451512 Free PMC article. Review.
Cited by
-
Salmonella Typhimurium and inflammation: a pathogen-centric affair.Nat Rev Microbiol. 2021 Nov;19(11):716-725. doi: 10.1038/s41579-021-00561-4. Epub 2021 May 19. Nat Rev Microbiol. 2021. PMID: 34012042 Free PMC article. Review.
-
Activation of the NLRP1B inflammasome by caspase-8.Commun Biol. 2024 Sep 17;7(1):1164. doi: 10.1038/s42003-024-06882-3. Commun Biol. 2024. PMID: 39289441 Free PMC article.
-
NLRP3 Inflammasome-Mediated Neuroinflammation and Related Mitochondrial Impairment in Parkinson's Disease.Neurosci Bull. 2023 May;39(5):832-844. doi: 10.1007/s12264-023-01023-y. Epub 2023 Feb 9. Neurosci Bull. 2023. PMID: 36757612 Free PMC article. Review.
-
Molecular mechanisms and roles of pyroptosis in acute lung injury.Chin Med J (Engl). 2022 Oct 20;135(20):2417-2426. doi: 10.1097/CM9.0000000000002425. Chin Med J (Engl). 2022. PMID: 36583860 Free PMC article.
-
Caspase-1 Inhibitor Reduces Pyroptosis Induced by Brain Death in Kidney.Front Surg. 2021 Nov 26;8:760989. doi: 10.3389/fsurg.2021.760989. eCollection 2021. Front Surg. 2021. PMID: 34901142 Free PMC article.
References
-
- Allam R, Lawlor KE, Yu EC, Mildenhall AL, Moujalled DM, Lewis RS, Ke F, Mason KD, White MJ, Stacey KJ, et al. 2014. Mitochondrial apoptosis is dispensable for NLRP3 inflammasome activation but non-apoptotic caspase-8 is required for inflammasome priming. EMBO Rep 15: 982–990. 10.15252/embr.201438463 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
