Adenoviral Infections in Singapore: Should New Antiviral Therapies and Vaccines Be Adopted?
- PMID: 31563943
- PMCID: PMC7107482
- DOI: 10.1093/infdis/jiz489
Adenoviral Infections in Singapore: Should New Antiviral Therapies and Vaccines Be Adopted?
Abstract
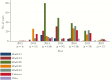
Background: A number of serious human adenovirus (HAdV) outbreaks have been recently reported: HAdV-B7 (Israel, Singapore, and USA), HAdV-B7d (USA and China), HAdV-D8, -D54, and -C2 (Japan), HAdV-B14p1 (USA, Europe, and China), and HAdV-B55 (China, Singapore, and France).
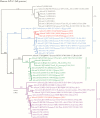
Methods: To understand the epidemiology of HAdV infections in Singapore, we studied 533 HAdV-positive clinical samples collected from 396 pediatric and 137 adult patients in Singapore from 2012 to 2018. Genome sequencing and phylogenetic analyses were performed to identify HAdV genotypes, clonal clusters, and recombinant or novel HAdVs.
Results: The most prevalent genotypes identified were HAdV-B3 (35.6%), HAdV-B7 (15.4%), and HAdV-E4 (15.2%). We detected 4 new HAdV-C strains and detected incursions with HAdV-B7 (odds ratio [OR], 14.6; 95% confidence interval [CI], 4.1-52.0) and HAdV-E4 (OR, 13.6; 95% CI, 3.9-46.7) among pediatric patients over time. In addition, immunocompromised patients (adjusted OR [aOR], 11.4; 95% CI, 3.8-34.8) and patients infected with HAdV-C2 (aOR, 8.5; 95% CI, 1.5-48.0), HAdV-B7 (aOR, 3.7; 95% CI, 1.2-10.9), or HAdV-E4 (aOR, 3.2; 95% CI, 1.1-8.9) were at increased risk for severe disease.
Conclusions: Singapore would benefit from more frequent studies of clinical HAdV genotypes to identify patients at risk for severe disease and help guide the use of new antiviral therapies, such as brincidofovir, and potential administration of HAdV 4 and 7 vaccine.
Keywords: adenovirus; genotyping; molecular epidemiology; pediatric disease; respiratory disease.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures

Similar articles
-
Adenovirus infection in children with acute lower respiratory tract infections in Beijing, China, 2007 to 2012.BMC Infect Dis. 2015 Oct 1;15:408. doi: 10.1186/s12879-015-1126-2. BMC Infect Dis. 2015. PMID: 26429778 Free PMC article.
-
Human Adenovirus Associated with Severe Respiratory Infection, Oregon, USA, 2013-2014.Emerg Infect Dis. 2016 Jun;22(6):1044-51. doi: 10.3201/eid2206.151898. Emerg Infect Dis. 2016. PMID: 27191834 Free PMC article.
-
Risk factor analysis and molecular epidemiology of respiratory adenovirus infections among children in northern Taiwan, 2009-2013.J Microbiol Immunol Infect. 2017 Aug;50(4):418-426. doi: 10.1016/j.jmii.2015.08.006. Epub 2015 Sep 9. J Microbiol Immunol Infect. 2017. PMID: 26454422
-
A review of 65 years of human adenovirus seroprevalence.Expert Rev Vaccines. 2019 Jun;18(6):597-613. doi: 10.1080/14760584.2019.1588113. Epub 2019 May 27. Expert Rev Vaccines. 2019. PMID: 31132024 Review.
-
Emergence and re-emergence of respiratory adenoviruses in the United States.Curr Opin Virol. 2019 Feb;34:63-69. doi: 10.1016/j.coviro.2018.12.004. Epub 2019 Jan 14. Curr Opin Virol. 2019. PMID: 30654272 Review.
Cited by
-
Patient hematology during hospitalization for viral pneumonia caused by SARS-CoV-2 and non-SARS-CoV-2 agents: a retrospective study.Eur J Med Res. 2021 May 14;26(1):45. doi: 10.1186/s40001-021-00515-9. Eur J Med Res. 2021. PMID: 33990223 Free PMC article.
-
Outbreak of severe acute respiratory infection in Southern Province, Sri Lanka in 2018: a cross-sectional study.BMJ Open. 2020 Nov 6;10(11):e040612. doi: 10.1136/bmjopen-2020-040612. BMJ Open. 2020. PMID: 33158834 Free PMC article.
-
Construction and Characterization of a Novel Recombinant Attenuated and Replication-Deficient Candidate Human Adenovirus Type 3 Vaccine: "Adenovirus Vaccine Within an Adenovirus Vector".Virol Sin. 2021 Jun;36(3):354-364. doi: 10.1007/s12250-020-00234-1. Epub 2020 May 26. Virol Sin. 2021. PMID: 32458297 Free PMC article.
-
Seroprevalence of Neutralizing Antibodies against Six Human Adenovirus Types Indicates the Low Level of Herd Immunity in Young Children from Guangzhou, China.Virol Sin. 2021 Jun;36(3):373-381. doi: 10.1007/s12250-020-00307-1. Epub 2020 Nov 9. Virol Sin. 2021. PMID: 33165772 Free PMC article.
-
Molecular Typing and Rapid Identification of Human Adenoviruses Associated With Respiratory Diseases Using Universal PCR and Sequencing Primers for the Three Major Capsid Genes: Penton Base, Hexon, and Fiber.Front Microbiol. 2022 May 12;13:911694. doi: 10.3389/fmicb.2022.911694. eCollection 2022. Front Microbiol. 2022. PMID: 35633710 Free PMC article.
References
-
- Kajon AE, Lu X, Erdman DD, et al. . Molecular epidemiology and brief history of emerging adenovirus 14-associated respiratory disease in the United States. J Infect Dis 2010; 202:93–103. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

