Generation of Norovirus-Specific T Cells From Human Donors With Extensive Cross-Reactivity to Variant Sequences: Implications for Immunotherapy
- PMID: 31562500
- PMCID: PMC7325618
- DOI: 10.1093/infdis/jiz491
Generation of Norovirus-Specific T Cells From Human Donors With Extensive Cross-Reactivity to Variant Sequences: Implications for Immunotherapy
Abstract
Background: Chronic norovirus infection in immunocompromised patients can be severe, and presently there is no effective treatment. Adoptive transfer of virus-specific T cells has proven to be safe and effective for the treatment of many viral infections, and this could represent a novel treatment approach for chronic norovirus infection. Hence, we sought to generate human norovirus-specific T cells (NSTs) that can recognize different viral sequences.
Methods: Norovirus-specific T cells were generated from peripheral blood of healthy donors by stimulation with overlapping peptide libraries spanning the entire coding sequence of the norovirus genome.
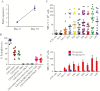
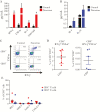
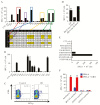
Results: We successfully generated T cells targeting multiple norovirus antigens with a mean 4.2 ± 0.5-fold expansion after 10 days. Norovirus-specific T cells comprised both CD4+ and CD8+ T cells that expressed markers for central memory and effector memory phenotype with minimal expression of coinhibitory molecules, and they were polyfunctional based on cytokine production. We identified novel CD4- and CD8-restricted immunodominant epitopes within NS6 and VP1 antigens. Furthermore, NSTs showed a high degree of cross-reactivity to multiple variant epitopes from clinical isolates.
Conclusions: Our findings identify immunodominant human norovirus T-cell epitopes and demonstrate that it is feasible to generate potent NSTs from third-party donors for use in antiviral immunotherapy.
Keywords: T cells; adoptive immunotherapy; norovirus; primary immunodeficiency; transplantation.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures

Similar articles
-
Identification of cross-reactive norovirus CD4+ T cell epitopes.J Virol. 2010 Sep;84(17):8530-8. doi: 10.1128/JVI.00727-10. Epub 2010 Jun 23. J Virol. 2010. PMID: 20573810 Free PMC article.
-
Generation of Zika virus-specific T cells from seropositive and virus-naïve donors for potential use as an autologous or "off-the-shelf" immunotherapeutic.Cytotherapy. 2019 Aug;21(8):840-855. doi: 10.1016/j.jcyt.2019.06.008. Epub 2019 Jul 4. Cytotherapy. 2019. PMID: 31279695 Free PMC article.
-
Human parainfluenza virus-3 can be targeted by rapidly ex vivo expanded T lymphocytes.Cytotherapy. 2016 Dec;18(12):1515-1524. doi: 10.1016/j.jcyt.2016.08.010. Epub 2016 Sep 28. Cytotherapy. 2016. PMID: 27692559 Free PMC article.
-
Human Epitopes Identified from Herpes Simplex Virus Tegument Protein VP11/12 (UL46) Recall Multifunctional Effector Memory CD4+ TEM Cells in Asymptomatic Individuals and Protect from Ocular Herpes Infection and Disease in "Humanized" HLA-DR Transgenic Mice.J Virol. 2020 Mar 17;94(7):e01991-19. doi: 10.1128/JVI.01991-19. Print 2020 Mar 17. J Virol. 2020. PMID: 31915285 Free PMC article.
-
Immunodominance of lytic cycle antigens in Epstein-Barr virus-specific CD4+ T cell preparations for therapy.PLoS One. 2007 Jul 4;2(7):e583. doi: 10.1371/journal.pone.0000583. PLoS One. 2007. PMID: 17611619 Free PMC article. Review.
Cited by
-
Pathogen-specific T Cells: Targeting Old Enemies and New Invaders in Transplantation and Beyond.Hemasphere. 2023 Jan 9;7(1):e809. doi: 10.1097/HS9.0000000000000809. eCollection 2023 Jan. Hemasphere. 2023. PMID: 36698615 Free PMC article. Review.
-
Noroviruses: Evolutionary Dynamics, Epidemiology, Pathogenesis, and Vaccine Advances-A Comprehensive Review.Vaccines (Basel). 2024 May 29;12(6):590. doi: 10.3390/vaccines12060590. Vaccines (Basel). 2024. PMID: 38932319 Free PMC article. Review.
-
Linear epitopes on the capsid protein of norovirus commonly elicit high antibody response among past-infected individuals.Virol J. 2023 Jun 6;20(1):115. doi: 10.1186/s12985-023-02087-y. Virol J. 2023. PMID: 37280660 Free PMC article.
-
Emerging trends in COVID-19 treatment: learning from inflammatory conditions associated with cellular therapies.Cytotherapy. 2020 Sep;22(9):474-481. doi: 10.1016/j.jcyt.2020.04.100. Epub 2020 May 7. Cytotherapy. 2020. PMID: 32565132 Free PMC article. Review.
-
Towards a Comprehensive Understanding of Human Norovirus Immunity.Cell Mol Gastroenterol Hepatol. 2020;10(2):422-423. doi: 10.1016/j.jcmgh.2020.04.019. Epub 2020 May 29. Cell Mol Gastroenterol Hepatol. 2020. PMID: 32479756 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

