Connective tissue fibroblasts from highly regenerative mammals are refractory to ROS-induced cellular senescence
- PMID: 31562333
- PMCID: PMC6764955
- DOI: 10.1038/s41467-019-12398-w
Connective tissue fibroblasts from highly regenerative mammals are refractory to ROS-induced cellular senescence
Abstract
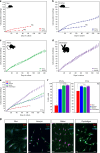
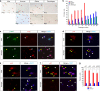
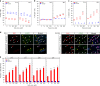
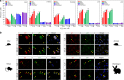
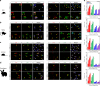
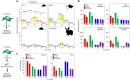
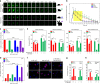
A surveillance system in mammals constantly monitors cell activity to protect against aberrant proliferation in response to damage, injury and oncogenic stress. Here we isolate and culture connective tissue fibroblasts from highly regenerative mammals (Acomys and Oryctolagus) to determine how these cells interpret signals that normally induce cellular senescence in non-regenerating mammals (Mus and Rattus). While H2O2 exposure substantially decreases cell proliferation and increases p53, p21, p16, and p19 in cells from mice and rats, cells from spiny mice and rabbits are highly resistant to H2O2. Quantifying oxygen consumption and mitochondrial stability, we demonstrate that increased intracellular H2O2 is rapidly detoxified in regenerating species, but overwhelms antioxidant scavenging in cells from non-regenerative mammals. However, pretreatment with N-acetylcysteine (NAC) protects mouse and rat cells from ROS-induced cellular senescence. Collectively, our results show that intrinsic cellular differences in stress-sensing mechanisms partially explain interspecific variation in regenerative ability.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
N-acetylcysteine protects alveolar epithelial cells from hydrogen peroxide-induced apoptosis through scavenging reactive oxygen species and suppressing c-Jun N-terminal kinase.Exp Lung Res. 2010 Aug;36(6):352-61. doi: 10.3109/01902141003678582. Exp Lung Res. 2010. PMID: 20653470
-
Proinflammatory cytokine-induced cellular senescence of biliary epithelial cells is mediated via oxidative stress and activation of ATM pathway: a culture study.Free Radic Res. 2008 Jul;42(7):625-32. doi: 10.1080/10715760802244768. Epub 2008 Jun 25. Free Radic Res. 2008. PMID: 18608517
-
Interferon-gamma induces cellular senescence through p53-dependent DNA damage signaling in human endothelial cells.Mech Ageing Dev. 2009 Mar;130(3):179-88. doi: 10.1016/j.mad.2008.11.004. Epub 2008 Nov 21. Mech Ageing Dev. 2009. PMID: 19071156
-
Hyperoside prevents oxidative damage induced by hydrogen peroxide in lung fibroblast cells via an antioxidant effect.Biochim Biophys Acta. 2008 Dec;1780(12):1448-57. doi: 10.1016/j.bbagen.2008.07.012. Epub 2008 Aug 15. Biochim Biophys Acta. 2008. PMID: 18761393
-
Protective effects of rosmarinic acid against hydrogen peroxide‑induced cellular senescence and the inflammatory response in normal human dermal fibroblasts.Mol Med Rep. 2017 Dec;16(6):9763-9769. doi: 10.3892/mmr.2017.7804. Epub 2017 Oct 17. Mol Med Rep. 2017. PMID: 29039587
Cited by
-
Simple Detection of Unstained Live Senescent Cells with Imaging Flow Cytometry.Cells. 2022 Aug 12;11(16):2506. doi: 10.3390/cells11162506. Cells. 2022. PMID: 36010584 Free PMC article.
-
Biodegradable hollow mesoporous organosilica nanotheranostics (HMON) for multi-mode imaging and mild photo-therapeutic-induced mitochondrial damage on gastric cancer.J Nanobiotechnology. 2020 Jul 20;18(1):99. doi: 10.1186/s12951-020-00653-y. J Nanobiotechnology. 2020. PMID: 32690085 Free PMC article.
-
Antibiotic Azithromycin inhibits brown/beige fat functionality and promotes obesity in human and rodents.Theranostics. 2022 Jan 1;12(3):1187-1203. doi: 10.7150/thno.63067. eCollection 2022. Theranostics. 2022. PMID: 35154482 Free PMC article.
-
Regeneration in the spiny mouse, Acomys, a new mammalian model.Curr Opin Genet Dev. 2020 Oct;64:31-36. doi: 10.1016/j.gde.2020.05.019. Epub 2020 Jun 26. Curr Opin Genet Dev. 2020. PMID: 32599302 Free PMC article. Review.
-
Hemoglobin induces oxidative stress and mitochondrial dysfunction in oligodendrocyte progenitor cells.Transl Res. 2021 May;231:13-23. doi: 10.1016/j.trsl.2021.01.005. Epub 2021 Jan 15. Transl Res. 2021. PMID: 33460824 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

