DNA vaccine based on conserved HA-peptides induces strong immune response and rapidly clears influenza virus infection from vaccinated pigs
- PMID: 31553755
- PMCID: PMC6760788
- DOI: 10.1371/journal.pone.0222201
DNA vaccine based on conserved HA-peptides induces strong immune response and rapidly clears influenza virus infection from vaccinated pigs
Abstract
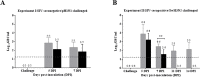
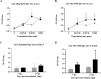
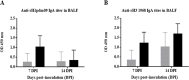
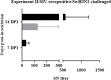
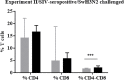
Swine influenza virus (SIVs) infections cause a significant economic impact to the pork industry. Moreover, pigs may act as mixing vessel favoring genome reassortment of diverse influenza viruses. Such an example is the pandemic H1N1 (pH1N1) virus that appeared in 2009, harboring a combination of gene segments from avian, pig and human lineages, which rapidly reached pandemic proportions. In order to confront and prevent these possible emergences as well as antigenic drift phenomena, vaccination remains of vital importance. The present work aimed to evaluate a new DNA influenza vaccine based on distinct conserved HA-peptides fused with flagellin and applied together with Diluvac Forte as adjuvant using a needle-free device (IntraDermal Application of Liquids, IDAL®). Two experimental pig studies were performed to test DNA-vaccine efficacy against SIVs in pigs. In the first experiment, SIV-seronegative pigs were vaccinated with VC4-flagellin DNA and intranasally challenged with a pH1N1. In the second study, VC4-flagellin DNA vaccine was employed in SIV-seropositive animals and challenged intranasally with an H3N2 SIV-isolate. Both experiments demonstrated a reduction in the viral shedding after challenge, suggesting vaccine efficacy against both the H1 and H3 influenza virus subtypes. In addition, the results proved that maternally derived antibodies (MDA) did not constitute an obstacle to the vaccine approach used. Moreover, elevated titers in antibodies both against H1 and H3 proteins in serum and in bronchoalveolar lavage fluids (BALFs) was detected in the vaccinated animals along with a markedly increased mucosal IgA response. Additionally, vaccinated animals developed stronger neutralizing antibodies in BALFs and higher inhibiting hemagglutination titers in sera against both the pH1N1 and H3N2 influenza viruses compared to unvaccinated, challenged-pigs. It is proposed that the described DNA-vaccine formulation could potentially be used as a multivalent vaccine against SIV infections.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Conserved HA-peptide NG34 formulated in pCMV-CTLA4-Ig reduces viral shedding in pigs after a heterosubtypic influenza virus SwH3N2 challenge.PLoS One. 2019 Mar 1;14(3):e0212431. doi: 10.1371/journal.pone.0212431. eCollection 2019. PLoS One. 2019. PMID: 30822308 Free PMC article.
-
Vaccination of pigs against swine influenza viruses by using an NS1-truncated modified live-virus vaccine.J Virol. 2006 Nov;80(22):11009-18. doi: 10.1128/JVI.00787-06. Epub 2006 Aug 30. J Virol. 2006. PMID: 16943300 Free PMC article.
-
A polyvalent influenza A DNA vaccine induces heterologous immunity and protects pigs against pandemic A(H1N1)pdm09 virus infection.Vaccine. 2013 Apr 26;31(18):2281-8. doi: 10.1016/j.vaccine.2013.02.061. Epub 2013 Mar 13. Vaccine. 2013. PMID: 23499598
-
[Swine influenza virus: evolution mechanism and epidemic characterization--a review].Wei Sheng Wu Xue Bao. 2009 Sep;49(9):1138-45. Wei Sheng Wu Xue Bao. 2009. PMID: 20030049 Review. Chinese.
-
Vaccine development for protecting swine against influenza virus.Anim Health Res Rev. 2012 Dec;13(2):181-95. doi: 10.1017/S1466252312000175. Anim Health Res Rev. 2012. PMID: 23253165 Review.
Cited by
-
Influenza A Virus in Swine: Epidemiology, Challenges and Vaccination Strategies.Front Vet Sci. 2020 Sep 22;7:647. doi: 10.3389/fvets.2020.00647. eCollection 2020. Front Vet Sci. 2020. PMID: 33195504 Free PMC article. Review.
-
Thermostable Vaccines in Veterinary Medicine: State of the Art and Opportunities to Be Seized.Vaccines (Basel). 2022 Feb 5;10(2):245. doi: 10.3390/vaccines10020245. Vaccines (Basel). 2022. PMID: 35214703 Free PMC article. Review.
-
Genetic diversification patterns in swine influenza A virus (H1N2) in vaccinated and nonvaccinated animals.Front Cell Infect Microbiol. 2023 Sep 15;13:1258321. doi: 10.3389/fcimb.2023.1258321. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37780850 Free PMC article.
-
Vaccination against swine influenza in pigs causes different drift evolutionary patterns upon swine influenza virus experimental infection and reduces the likelihood of genomic reassortments.Front Cell Infect Microbiol. 2023 Mar 13;13:1111143. doi: 10.3389/fcimb.2023.1111143. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 36992684 Free PMC article.
-
Evolution of Swine Influenza Virus H3N2 in Vaccinated and Nonvaccinated Pigs after Previous Natural H1N1 Infection.Viruses. 2022 Sep 10;14(9):2008. doi: 10.3390/v14092008. Viruses. 2022. PMID: 36146814 Free PMC article.
References
-
- Kothalawala H, Toussaint MJM, Gruys E. An overview of swine influenza. Vet Q. 2006;28: 46–53. Available: http://www.ncbi.nlm.nih.gov/pubmed/16841566 - PubMed
-
- Gorres JP, Lager KM, Kong WP, Royals M, Todd JP, Vincent AL, et al. DNA vaccination elicits protective immune responses against pandemic and classic swine influenza viruses in pigs. Clin Vaccine Immunol. American Society for Microbiology (ASM); 2011;18: 1987–1995. Available: http://www.ncbi.nlm.nih.gov/pubmed/21918118 10.1128/CVI.05171-11 - DOI - PMC - PubMed
-
- Martín-Valls GE, Simon-Grifé M, van Boheemen S, de Graaf M, Bestebroer TM, Busquets N, et al. Phylogeny of Spanish swine influenza viruses isolated from respiratory disease outbreaks and evolution of swine influenza virus within an endemically infected farm. Vet Microbiol. Elsevier; 2014;170: 266–277. Available: https://www-sciencedirect-com.are.uab.cat/science/article/pii/S037811351... 10.1016/j.vetmic.2014.02.031 - DOI - PubMed
-
- Alexander DJ, Brown IH. Recent zoonoses caused by influenza A viruses. Rev Sci Tech. 2000;19: 197–225. Available: http://www.ncbi.nlm.nih.gov/pubmed/11189716 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

