Engineering a Genetically Encoded Magnetic Protein Crystal
- PMID: 31552740
- PMCID: PMC7265822
- DOI: 10.1021/acs.nanolett.9b02266
Engineering a Genetically Encoded Magnetic Protein Crystal
Abstract
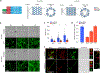
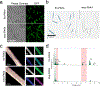
Magnetogenetics is a new field that leverages genetically encoded proteins and protein assemblies that are sensitive to magnetic fields to study and manipulate cell behavior. Theoretical studies show that many proposed magnetogenetic proteins do not contain enough iron to generate substantial magnetic forces. Here, we have engineered a genetically encoded ferritin-containing protein crystal that grows inside mammalian cells. Each of these crystals contains more than 10 million ferritin subunits and is capable of mineralizing substantial amounts of iron. When isolated from cells and loaded with iron in vitro, these crystals generate magnetic forces that are 9 orders of magnitude larger than the forces from the single ferritin cages used in previous studies. These protein crystals are attracted to an applied magnetic field and move toward magnets even when internalized into cells. While additional studies are needed to realize the full potential of magnetogenetics, these results demonstrate the feasibility of engineering protein assemblies for magnetic sensing.
Keywords: Magnetogenetics; ferritin; magnetic; protein crystal.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
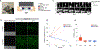

Similar articles
-
Possible magneto-mechanical and magneto-thermal mechanisms of ion channel activation in magnetogenetics.Elife. 2019 Aug 2;8:e45807. doi: 10.7554/eLife.45807. Elife. 2019. PMID: 31373554 Free PMC article.
-
Genetically programmed superparamagnetic behavior of mammalian cells.J Biotechnol. 2012 Dec 31;162(2-3):237-45. doi: 10.1016/j.jbiotec.2012.09.019. Epub 2012 Oct 2. J Biotechnol. 2012. PMID: 23036923
-
Ferritin at different iron loading: From biological to nanotechnological applications.Int J Biol Macromol. 2024 Sep;276(Pt 2):133812. doi: 10.1016/j.ijbiomac.2024.133812. Epub 2024 Jul 19. Int J Biol Macromol. 2024. PMID: 39032902
-
The Ferritin-like superfamily: Evolution of the biological iron storeman from a rubrerythrin-like ancestor.Biochim Biophys Acta. 2010 Aug;1800(8):691-705. doi: 10.1016/j.bbagen.2010.05.010. Epub 2010 May 27. Biochim Biophys Acta. 2010. PMID: 20553812 Review.
-
Magnetogenetics as a promising tool for controlling cellular signaling pathways.J Nanobiotechnology. 2024 Jun 10;22(1):327. doi: 10.1186/s12951-024-02616-z. J Nanobiotechnology. 2024. PMID: 38858689 Free PMC article. Review.
Cited by
-
Engineering mammalian living materials towards clinically relevant therapeutics.EBioMedicine. 2021 Dec;74:103717. doi: 10.1016/j.ebiom.2021.103717. Epub 2021 Nov 25. EBioMedicine. 2021. PMID: 34839265 Free PMC article. Review.
-
Magnetic protein aggregates generated by supramolecular assembly of ferritin cages - a modular strategy for the immobilization of enzymes.Front Bioeng Biotechnol. 2024 Oct 23;12:1478198. doi: 10.3389/fbioe.2024.1478198. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39512655 Free PMC article.
-
A Review of Electromagnetic Fields in Cellular Interactions and Cacao Bean Fermentation.Foods. 2024 Sep 26;13(19):3058. doi: 10.3390/foods13193058. Foods. 2024. PMID: 39410093 Free PMC article. Review.
-
From an obliquely falling rod in a viscous fluid to the motion of suspended magnetic bead chains that are driven by a gradient magnetic field and that make an arbitrary angle with the magnetic force vector: A Stokes flow study.PLoS One. 2024 Apr 16;19(4):e0301852. doi: 10.1371/journal.pone.0301852. eCollection 2024. PLoS One. 2024. PMID: 38625980 Free PMC article.
-
One-Pot Synthesis of Multiple Stimuli-Responsive Magnetic Nanomaterials Based on the Biomineralization of Elastin-like Polypeptides.ACS Omega. 2021 Oct 13;6(42):27946-27954. doi: 10.1021/acsomega.1c03821. eCollection 2021 Oct 26. ACS Omega. 2021. PMID: 34722994 Free PMC article.
References
-
- Liße D; Monzel C; Vicario C; Manzi J; Maurin I; Coppey M; Piehler J; Dahan M Engineered Ferritin for Magnetogenetic Manipulation of Proteins and Organelles Inside Living Cells. Adv. Mater 2017, 29 (42), 1700189. - PubMed
-
- Chen R; Romero G; Christiansen MG; Mohr A; Anikeeva P Wireless Magnetothermal Deep Brain Stimulation. Science 2015, 347 (6229), 1477–1480. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

