Towards a Better Understanding of Cohesin Mutations in AML
- PMID: 31552185
- PMCID: PMC6746210
- DOI: 10.3389/fonc.2019.00867
Towards a Better Understanding of Cohesin Mutations in AML
Abstract
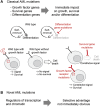
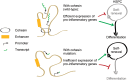
Classical driver mutations in acute myeloid leukemia (AML) typically affect regulators of cell proliferation, differentiation, and survival. The selective advantage of increased proliferation, improved survival, and reduced differentiation on leukemia progression is immediately obvious. Recent large-scale sequencing efforts have uncovered numerous novel AML-associated mutations. Interestingly, a substantial fraction of the most frequently mutated genes encode general regulators of transcription and chromatin state. Understanding the selective advantage conferred by these mutations remains a major challenge. A striking example are mutations in genes of the cohesin complex, a major regulator of three-dimensional genome organization. Several landmark studies have shown that cohesin mutations perturb the balance between self-renewal and differentiation of hematopoietic stem and progenitor cells (HSPC). Emerging data now begin to uncover the molecular mechanisms that underpin this phenotype. Among these mechanisms is a role for cohesin in the control of inflammatory responses in HSPCs and myeloid cells. Inflammatory signals limit HSPC self-renewal and drive HSPC differentiation. Consistent with this, cohesin mutations promote resistance to inflammatory signals, and may provide a selective advantage for AML progression. In this review, we discuss recent progress in understanding cohesin mutations in AML, and speculate whether vulnerabilities associated with these mutations could be exploited therapeutically.
Keywords: AML; cohesin; hematopoiesis; inflammation; interferon; leukemia.
Figures
Similar articles
-
DOT1L inhibitors block abnormal self-renewal induced by cohesin loss.Sci Rep. 2021 Mar 31;11(1):7288. doi: 10.1038/s41598-021-86646-9. Sci Rep. 2021. PMID: 33790356 Free PMC article.
-
The cohesin subunit Rad21 is a negative regulator of hematopoietic self-renewal through epigenetic repression of Hoxa7 and Hoxa9.Leukemia. 2017 Mar;31(3):712-719. doi: 10.1038/leu.2016.240. Epub 2016 Aug 24. Leukemia. 2017. PMID: 27554164 Free PMC article.
-
The role of mutations in the cohesin complex in acute myeloid leukemia.Int J Hematol. 2017 Jan;105(1):31-36. doi: 10.1007/s12185-016-2119-7. Epub 2016 Oct 28. Int J Hematol. 2017. PMID: 27796738 Free PMC article. Review.
-
Characteristics of Cohesin Mutation in Acute Myeloid Leukemia and Its Clinical Significance.Front Oncol. 2021 Apr 13;11:579881. doi: 10.3389/fonc.2021.579881. eCollection 2021. Front Oncol. 2021. PMID: 33928020 Free PMC article. Review.
-
Cohesin Mutations in Myeloid Malignancies.Trends Cancer. 2017 Apr;3(4):282-293. doi: 10.1016/j.trecan.2017.02.006. Trends Cancer. 2017. PMID: 28626802 Free PMC article. Review.
Cited by
-
Genetic variation as a long-distance modulator of RAD21 expression in humans.Sci Rep. 2022 Jul 29;12(1):13035. doi: 10.1038/s41598-022-15081-1. Sci Rep. 2022. PMID: 35906355 Free PMC article.
-
Novel Molecular Insights into Leukemic Evolution of Myeloproliferative Neoplasms: A Single Cell Perspective.Int J Mol Sci. 2022 Dec 3;23(23):15256. doi: 10.3390/ijms232315256. Int J Mol Sci. 2022. PMID: 36499582 Free PMC article. Review.
-
The glucocorticoid receptor associates with the cohesin loader NIPBL to promote long-range gene regulation.Sci Adv. 2022 Apr;8(13):eabj8360. doi: 10.1126/sciadv.abj8360. Epub 2022 Mar 30. Sci Adv. 2022. PMID: 35353576 Free PMC article.
-
Down syndrome and leukemia: from basic mechanisms to clinical advances.Haematologica. 2023 Oct 1;108(10):2570-2581. doi: 10.3324/haematol.2023.283225. Haematologica. 2023. PMID: 37439336 Free PMC article. Review.
-
Molecular Systems Architecture of Interactome in the Acute Myeloid Leukemia Microenvironment.Cancers (Basel). 2022 Feb 1;14(3):756. doi: 10.3390/cancers14030756. Cancers (Basel). 2022. PMID: 35159023 Free PMC article. Review.

