A Conserved Tyrosine Residue in Slitrk3 Carboxyl-Terminus Is Critical for GABAergic Synapse Development
- PMID: 31551708
- PMCID: PMC6746929
- DOI: 10.3389/fnmol.2019.00213
A Conserved Tyrosine Residue in Slitrk3 Carboxyl-Terminus Is Critical for GABAergic Synapse Development
Abstract
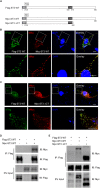
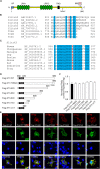
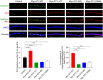
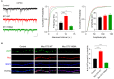
Single-passing transmembrane protein, Slitrk3 (Slit and Trk-like family member 3, ST3), is a synaptic cell adhesion molecule highly expressed at inhibitory synapses. Recent studies have shown that ST3, through its extracellular domain, selectively regulates inhibitory synapse development via the trans-synaptic interaction with presynaptic cell adhesion molecule, receptor protein tyrosine phosphatase δ (PTPδ) and the cis-interaction with postsynaptic cell adhesion molecule, Neuroligin 2 (NL2). However, little is known about the physiological function of ST3 intracellular, carboxyl (C)-terminal region. Here we report that in heterologous cells, ST3 C-terminus is not required for ST3 homo-dimerization and trafficking to the cell surface. In contrast, in hippocampal neurons, ST3 C-terminus, more specifically, the conserved tyrosine Y969 (in mice), is critical for GABAergic synapse development. Indeed, overexpression of ST3 Y969A mutant markedly reduced the gephyrin puncta density and GABAergic transmission in hippocampal neurons. In addition, single-cell genetic deletion of ST3 strongly impaired GABAergic transmission. Importantly, wild-type (WT) ST3, but not the ST3 Y969A mutant, could fully rescue GABAergic transmission deficits in neurons lacking endogenous ST3, confirming a critical role of Y969 in the regulation of inhibitory synapses. Taken together, our data identify a single critical residue in ST3 C-terminus that is important for GABAergic synapse development and function.
Keywords: GABAergic synapse; GABAergic synapse development; Slitrk3; cell adhesion molecule; gephyrin; hippocampus; inhibition; tyrosine.
Figures
Similar articles
-
Molecular Dissection of Neuroligin 2 and Slitrk3 Reveals an Essential Framework for GABAergic Synapse Development.Neuron. 2017 Nov 15;96(4):808-826.e8. doi: 10.1016/j.neuron.2017.10.003. Epub 2017 Oct 26. Neuron. 2017. PMID: 29107521 Free PMC article.
-
Selective control of inhibitory synapse development by Slitrk3-PTPδ trans-synaptic interaction.Nat Neurosci. 2012 Jan 29;15(3):389-98, S1-2. doi: 10.1038/nn.3040. Nat Neurosci. 2012. PMID: 22286174 Free PMC article.
-
ErbB4 promotes inhibitory synapse formation by cell adhesion, independent of its kinase activity.Transl Psychiatry. 2021 Jun 29;11(1):361. doi: 10.1038/s41398-021-01485-6. Transl Psychiatry. 2021. PMID: 34226493 Free PMC article.
-
Differential role of GABAA receptors and neuroligin 2 for perisomatic GABAergic synapse formation in the hippocampus.Brain Struct Funct. 2017 Dec;222(9):4149-4161. doi: 10.1007/s00429-017-1462-7. Epub 2017 Jun 22. Brain Struct Funct. 2017. PMID: 28643105
-
Activity-dependent development of GABAergic synapses.Brain Res. 2019 Mar 15;1707:18-26. doi: 10.1016/j.brainres.2018.11.014. Epub 2018 Nov 12. Brain Res. 2019. PMID: 30439352 Review.
Cited by
-
SLITRK2 variants associated with neurodevelopmental disorders impair excitatory synaptic function and cognition in mice.Nat Commun. 2022 Jul 15;13(1):4112. doi: 10.1038/s41467-022-31566-z. Nat Commun. 2022. PMID: 35840571 Free PMC article.
-
Human mutations in SLITRK3 implicated in GABAergic synapse development in mice.Front Mol Neurosci. 2024 Mar 1;17:1222935. doi: 10.3389/fnmol.2024.1222935. eCollection 2024. Front Mol Neurosci. 2024. PMID: 38495551 Free PMC article.
References
-
- Dani V. S., Chang Q., Maffei A., Turrigiano G. G., Jaenisch R., Nelson S. B. (2005). Reduced cortical activity due to a shift in the balance between excitation and inhibition in a mouse model of rett syndrome. Proc. Natl. Acad. Sci. U.S.A. 102 12560–12565. 10.1073/pnas.0506071102 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

