Cubic and hexagonal liquid crystal gels for ocular delivery with enhanced effect of pilocarpine nitrate on anti-glaucoma treatment
- PMID: 31544551
- PMCID: PMC6764361
- DOI: 10.1080/10717544.2019.1667451
Cubic and hexagonal liquid crystal gels for ocular delivery with enhanced effect of pilocarpine nitrate on anti-glaucoma treatment
Abstract
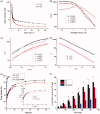
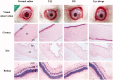
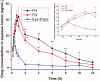
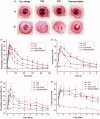
The objective of this work was to investigate phytantriol-based liquid crystal (LC) gels including cubic (Q2) and hexagonal (H2) phase for ocular delivery of pilocarpine nitrate (PN) to treat glaucoma. The gels were produced by a vortex method and confirmed by crossed polarized light microscopy, small-angle X-ray scattering, and rheological measurements. Moreover, the release behaviors and permeation results of PN from the gels were estimated using in vitro studies. Finally, the anti-glaucoma effect of LC gels was evaluated by in vivo animal experiments. The inner structure of the gels was Pn3m-type Q2 and H2 phase, and both of them showed pseudoplastic fluid properties based on characterization techniques. In vitro release profiles suggested that PN could be sustainably released from LC gels within 48 h. Compared with eye drops, Q2 and H2 gel produces a 5.25-fold and 6.23-fold increase in the Papp value (p < .05), respectively, leading to a significant enhancement of corneal penetration. Furthermore, a good biocompatibility and longer residence time on precorneal for LC gels confirmed by in vivo animal experiment. Pharmacokinetic studies showed that LC gels could maintain PN concentration in aqueous humor for at least 12 h after administration and remarkably improve the bioavailability of drug. Additionally, in vivo pharmacodynamics studies indicated that LC gels had a more significant intraocular pressure-lowering and miotic effect compared to eye drops. These research findings hinted that LC gels would be a promising pharmaceutical strategy for ocular application to enhance the efficacy of anti-glaucoma.
Keywords: IOP-lowering; Phytantriol; anti-glaucoma; liquid crystal gels; ocular delivery.
Figures


Similar articles
-
A Novel Phytantriol-Based Lyotropic Liquid Crystalline Gel for Efficient Ophthalmic Delivery of Pilocarpine Nitrate.AAPS PharmSciTech. 2019 Jan 2;20(1):32. doi: 10.1208/s12249-018-1248-0. AAPS PharmSciTech. 2019. PMID: 30603986
-
Self-assembled hexagonal liquid crystalline gels as novel ocular formulation with enhanced topical delivery of pilocarpine nitrate.Int J Pharm. 2019 May 1;562:31-41. doi: 10.1016/j.ijpharm.2019.02.033. Epub 2019 Mar 14. Int J Pharm. 2019. PMID: 30878587
-
A potential carrier based on liquid crystal nanoparticles for ophthalmic delivery of pilocarpine nitrate.Int J Pharm. 2013 Oct 15;455(1-2):75-84. doi: 10.1016/j.ijpharm.2013.07.057. Epub 2013 Jul 31. Int J Pharm. 2013. PMID: 23916822
-
Chitosan-Based In Situ Gels for Ocular Delivery of Therapeutics: A State-of-the-Art Review.Mar Drugs. 2018 Oct 9;16(10):373. doi: 10.3390/md16100373. Mar Drugs. 2018. PMID: 30304825 Free PMC article. Review.
-
[Design of Novel Ophthalmic Formulation Containing Drug Nanoparticles and Its Usefulness as Anti-glaucoma Drugs].Yakugaku Zasshi. 2016;136(10):1385-1390. doi: 10.1248/yakushi.16-00089. Yakugaku Zasshi. 2016. PMID: 27725388 Review. Japanese.
Cited by
-
A liquid crystal in situ gel based on rotigotine for the treatment of Parkinson's disease.Drug Deliv Transl Res. 2024 Apr;14(4):1048-1062. doi: 10.1007/s13346-023-01449-x. Epub 2023 Oct 24. Drug Deliv Transl Res. 2024. PMID: 37875660
-
Structure, Merits, Gel Formation, Gel Preparation and Functions of Konjac Glucomannan and Its Application in Aquatic Food Preservation.Foods. 2023 Mar 13;12(6):1215. doi: 10.3390/foods12061215. Foods. 2023. PMID: 36981142 Free PMC article. Review.
-
Recent Advances in Ocular Drug Delivery: Insights into Lyotropic Liquid Crystals.Pharmaceuticals (Basel). 2024 Oct 2;17(10):1315. doi: 10.3390/ph17101315. Pharmaceuticals (Basel). 2024. PMID: 39458956 Free PMC article. Review.
-
Fabrication of Transgelosomes for Enhancing the Ocular Delivery of Acetazolamide: Statistical Optimization, In Vitro Characterization, and In Vivo Study.Pharmaceutics. 2020 May 20;12(5):465. doi: 10.3390/pharmaceutics12050465. Pharmaceutics. 2020. PMID: 32443679 Free PMC article.
-
Nanostructure in Amphiphile-Based Deep Eutectic Solvents.Langmuir. 2023 Nov 28;39(47):16776-16784. doi: 10.1021/acs.langmuir.3c02105. Epub 2023 Nov 15. Langmuir. 2023. PMID: 37965899 Free PMC article.
References
-
- Adams CM, Stacy R, Rangaswamy N, et al. (2018). Glaucoma – next generation therapeutics: impossible to possible. Pharm Res 36:25. - PubMed
-
- Almeida H, Amaral MH, Lobão P, et al. (2014). In situ gelling systems: a strategy to improve the bioavailability of ophthalmic pharmaceutical formulations. Drug Discov Today 19:400–12. - PubMed
-
- Barauskas J, Landh T (2003). Phase behavior of the phytantriol/water system. Langmuir 19:9562.
-
- Boyd BJ, Whittaker DV, Khoo SM, et al. (2006). Lyotropic liquid crystalline phases formed from glycerate surfactants as sustained release drug delivery systems. Int J Pharm 309:218–26. - PubMed
-
- Chou SF, Luo LJ, Lai JY (2016). Gallic acid grafting effect on delivery performance and antiglaucoma efficacy of antioxidant-functionalized intracameral pilocarpine carriers. Acta Biomater 38:116–28. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
