Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease
- PMID: 31542391
- PMCID: PMC6796530
- DOI: 10.1016/j.ebiom.2019.08.069
Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease
Erratum in
-
Corrigendum to 'Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease' EBioMedicine 47 (2019) 446-456.EBioMedicine. 2020 Feb;52:102595. doi: 10.1016/j.ebiom.2019.12.004. Epub 2020 Jan 23. EBioMedicine. 2020. PMID: 31982828 Free PMC article. No abstract available.
Abstract
Background: Senescent cells, which can release factors that cause inflammation and dysfunction, the senescence-associated secretory phenotype (SASP), accumulate with ageing and at etiological sites in multiple chronic diseases. Senolytics, including the combination of Dasatinib and Quercetin (D + Q), selectively eliminate senescent cells by transiently disabling pro-survival networks that defend them against their own apoptotic environment. In the first clinical trial of senolytics, D + Q improved physical function in patients with idiopathic pulmonary fibrosis (IPF), a fatal senescence-associated disease, but to date, no peer-reviewed study has directly demonstrated that senolytics decrease senescent cells in humans.
Methods: In an open label Phase 1 pilot study, we administered 3 days of oral D 100 mg and Q 1000 mg to subjects with diabetic kidney disease (N = 9; 68·7 ± 3·1 years old; 2 female; BMI:33·9 ± 2·3 kg/m2; eGFR:27·0 ± 2·1 mL/min/1·73m2). Adipose tissue, skin biopsies, and blood were collected before and 11 days after completing senolytic treatment. Senescent cell and macrophage/Langerhans cell markers and circulating SASP factors were assayed.
Findings: D + Q reduced adipose tissue senescent cell burden within 11 days, with decreases in p16INK4A-and p21CIP1-expressing cells, cells with senescence-associated β-galactosidase activity, and adipocyte progenitors with limited replicative potential. Adipose tissue macrophages, which are attracted, anchored, and activated by senescent cells, and crown-like structures were decreased. Skin epidermal p16INK4A+ and p21CIP1+ cells were reduced, as were circulating SASP factors, including IL-1α, IL-6, and MMPs-9 and -12.
Interpretation: "Hit-and-run" treatment with senolytics, which in the case of D + Q have elimination half-lives <11 h, significantly decreases senescent cell burden in humans. FUND: NIH and Foundations. ClinicalTrials.gov Identifier: NCT02848131. Senescence, Frailty, and Mesenchymal Stem Cell Functionality in Chronic Kidney Disease: Effect of Senolytic Agents.
Keywords: Cellular senescence; Dasatinib; Diabetic kidney disease; Quercetin; Senescence-associated secretory phenotype; Senolytics.
Copyright © 2019. Published by Elsevier B.V.
Conflict of interest statement
J.L.K., T.T., Y.Z., and N.K.L. have a financial interest related to this research. Patents on senolytic drugs are held by Mayo Clinic. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and was conducted in compliance with Mayo Clinic Conflict of Interest policies. No conflicts of interest, financial or otherwise, are declared by the other authors.
Figures
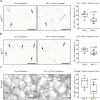

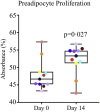

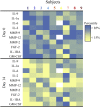
Comment in
-
Targeting senescent cells in ageing-related endocrine diseases.Nat Rev Endocrinol. 2023 Jul;19(7):382. doi: 10.1038/s41574-023-00848-x. Nat Rev Endocrinol. 2023. PMID: 37173439 No abstract available.
Similar articles
-
Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study.EBioMedicine. 2019 Feb;40:554-563. doi: 10.1016/j.ebiom.2018.12.052. Epub 2019 Jan 5. EBioMedicine. 2019. PMID: 30616998 Free PMC article. Clinical Trial.
-
Senolytic drugs, dasatinib and quercetin, attenuate adipose tissue inflammation, and ameliorate metabolic function in old age.Aging Cell. 2023 Feb;22(2):e13767. doi: 10.1111/acel.13767. Epub 2023 Jan 13. Aging Cell. 2023. PMID: 36637079 Free PMC article.
-
Targeting senescent cells alleviates obesity-induced metabolic dysfunction.Aging Cell. 2019 Jun;18(3):e12950. doi: 10.1111/acel.12950. Epub 2019 Mar 25. Aging Cell. 2019. PMID: 30907060 Free PMC article.
-
Senolytic drugs: from discovery to translation.J Intern Med. 2020 Nov;288(5):518-536. doi: 10.1111/joim.13141. Epub 2020 Aug 4. J Intern Med. 2020. PMID: 32686219 Free PMC article. Review.
-
Cellular Senescence in Diabetes Mellitus: Distinct Senotherapeutic Strategies for Adipose Tissue and Pancreatic β Cells.Front Endocrinol (Lausanne). 2022 Mar 31;13:869414. doi: 10.3389/fendo.2022.869414. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35432205 Free PMC article. Review.
Cited by
-
High salt diet accelerates skin aging in wistar rats: an 8-week investigation of cell cycle inhibitors, SASP markers, and oxidative stress.Front Bioeng Biotechnol. 2024 Oct 11;12:1450626. doi: 10.3389/fbioe.2024.1450626. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39465002 Free PMC article.
-
Senolytics prevent mt-DNA-induced inflammation and promote the survival of aged organs following transplantation.Nat Commun. 2020 Aug 27;11(1):4289. doi: 10.1038/s41467-020-18039-x. Nat Commun. 2020. PMID: 32855397 Free PMC article.
-
Declined adipogenic potential of senescent MSCs due to shift in insulin signaling and altered exosome cargo.Front Cell Dev Biol. 2022 Nov 17;10:1050489. doi: 10.3389/fcell.2022.1050489. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36467400 Free PMC article.
-
Quercetin Intake and Absolute Telomere Length in Patients with Type 2 Diabetes Mellitus: Novel Findings from a Randomized Controlled Before-and-After Study.Pharmaceuticals (Basel). 2024 Aug 29;17(9):1136. doi: 10.3390/ph17091136. Pharmaceuticals (Basel). 2024. PMID: 39338301 Free PMC article.
-
Dietary restriction in senolysis and prevention and treatment of disease.Crit Rev Food Sci Nutr. 2024;64(16):5242-5268. doi: 10.1080/10408398.2022.2153355. Epub 2022 Dec 9. Crit Rev Food Sci Nutr. 2024. PMID: 36484738 Free PMC article. Review.
References
-
- Hayflick L., Moorehead P. The serial cultivation of human diploid strains. Exp Cell Res. 1961;25:585–621. - PubMed
-
- Kirkland J.L., Hollenberg C.H., Gillon W.S. Age, anatomic site, and the replication and differentiation of adipocyte precursors. Am J Physiol. 1990;258 [C206-C10] - PubMed
Web Reference 1
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

