eIF5B gates the transition from translation initiation to elongation
- PMID: 31534220
- PMCID: PMC6763361
- DOI: 10.1038/s41586-019-1561-0
eIF5B gates the transition from translation initiation to elongation
Abstract
Translation initiation determines both the quantity and identity of the protein that is encoded in an mRNA by establishing the reading frame for protein synthesis. In eukaryotic cells, numerous translation initiation factors prepare ribosomes for polypeptide synthesis; however, the underlying dynamics of this process remain unclear1,2. A central question is how eukaryotic ribosomes transition from translation initiation to elongation. Here we use in vitro single-molecule fluorescence microscopy approaches in a purified yeast Saccharomyces cerevisiae translation system to monitor directly, in real time, the pathways of late translation initiation and the transition to elongation. This transition was slower in our eukaryotic system than that reported for Escherichia coli3-5. The slow entry to elongation was defined by a long residence time of eukaryotic initiation factor 5B (eIF5B) on the 80S ribosome after the joining of individual ribosomal subunits-a process that is catalysed by this universally conserved initiation factor. Inhibition of the GTPase activity of eIF5B after the joining of ribosomal subunits prevented the dissociation of eIF5B from the 80S complex, thereby preventing elongation. Our findings illustrate how the dissociation of eIF5B serves as a kinetic checkpoint for the transition from initiation to elongation, and how its release may be governed by a change in the conformation of the ribosome complex that triggers GTP hydrolysis.
Conflict of interest statement
The authors declare no competing interests. Readers are welcome to comment on the online version of the paper.
Figures








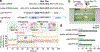


Similar articles
-
Structural basis for the transition from translation initiation to elongation by an 80S-eIF5B complex.Nat Commun. 2020 Oct 6;11(1):5003. doi: 10.1038/s41467-020-18829-3. Nat Commun. 2020. PMID: 33024099 Free PMC article.
-
Coupled release of eukaryotic translation initiation factors 5B and 1A from 80S ribosomes following subunit joining.Mol Cell Biol. 2007 Mar;27(6):2384-97. doi: 10.1128/MCB.02254-06. Epub 2007 Jan 22. Mol Cell Biol. 2007. PMID: 17242201 Free PMC article.
-
The Interaction between the Ribosomal Stalk Proteins and Translation Initiation Factor 5B Promotes Translation Initiation.Mol Cell Biol. 2018 Jul 30;38(16):e00067-18. doi: 10.1128/MCB.00067-18. Print 2018 Aug 15. Mol Cell Biol. 2018. PMID: 29844065 Free PMC article.
-
Universal translation initiation factor IF2/eIF5B.Cold Spring Harb Symp Quant Biol. 2001;66:417-24. doi: 10.1101/sqb.2001.66.417. Cold Spring Harb Symp Quant Biol. 2001. PMID: 12762044 Review. No abstract available.
-
Structural insights into eukaryotic ribosomes and the initiation of translation.Curr Opin Struct Biol. 2012 Dec;22(6):768-77. doi: 10.1016/j.sbi.2012.07.010. Epub 2012 Aug 10. Curr Opin Struct Biol. 2012. PMID: 22889726 Review.
Cited by
-
Structural Insights into the Mammalian Late-Stage Initiation Complexes.Cell Rep. 2020 Apr 7;31(1):107497. doi: 10.1016/j.celrep.2020.03.061. Cell Rep. 2020. PMID: 32268096 Free PMC article.
-
Sordarin bound eEF2 unlocks spontaneous forward and reverse translocation on CrPV IRES.Nucleic Acids Res. 2023 Jul 21;51(13):6999-7013. doi: 10.1093/nar/gkad476. Nucleic Acids Res. 2023. PMID: 37283061 Free PMC article.
-
mRNA- and factor-driven dynamic variability controls eIF4F-cap recognition for translation initiation.Nucleic Acids Res. 2022 Aug 12;50(14):8240-8261. doi: 10.1093/nar/gkac631. Nucleic Acids Res. 2022. PMID: 35871304 Free PMC article.
-
Metabolite-based cell sorting workflow for identifying microbes producing carbonyls in tobacco leaves.Appl Microbiol Biotechnol. 2022 Jun;106(11):4199-4209. doi: 10.1007/s00253-022-11982-3. Epub 2022 May 23. Appl Microbiol Biotechnol. 2022. PMID: 35599257
-
Effects of Oxidative Stress on Protein Translation: Implications for Cardiovascular Diseases.Int J Mol Sci. 2020 Apr 11;21(8):2661. doi: 10.3390/ijms21082661. Int J Mol Sci. 2020. PMID: 32290431 Free PMC article. Review.
References
-
- Hinnebusch AG The scanning mechanism of eukaryotic translation initiation. Annu. Rev. Biochem 83, 779–812 (2014). - PubMed
Methods-only References
-
- Yin J, Lin AJ, Golan DE & Walsh CT Site-specific protein labeling by Sfp phosphopantetheinyl transferase. Nat. Protoc 1, 280–285 (2006). - PubMed
-
- Maniatis T, Fritsch EF & Sambrook J Molecular cloning: a laboratory manual. (Cold Spring Harbor Laboratory Press, 1982).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

