The Synaptic Organization of Layer 6 Circuits Reveals Inhibition as a Major Output of a Neocortical Sublamina
- PMID: 31533036
- PMCID: PMC6941480
- DOI: 10.1016/j.celrep.2019.08.048
The Synaptic Organization of Layer 6 Circuits Reveals Inhibition as a Major Output of a Neocortical Sublamina
Abstract
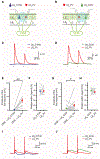
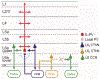
The canonical cortical microcircuit has principally been defined by interlaminar excitatory connections among the six layers of the neocortex. However, excitatory neurons in layer 6 (L6), a layer whose functional organization is poorly understood, form relatively rare synaptic connections with other cortical excitatory neurons. Here, we show that the vast majority of parvalbumin inhibitory neurons in a sublamina within L6 send axons through the cortical layers toward the pia. These interlaminar inhibitory neurons receive local synaptic inputs from both major types of L6 excitatory neurons and receive stronger input from thalamocortical afferents than do neighboring pyramidal neurons. The distribution of these interlaminar interneurons and their synaptic connectivity further support a functional subdivision within the standard six layers of the cortex. Positioned to integrate local and long-distance inputs in this sublayer, these interneurons generate an inhibitory interlaminar output. These findings call for a revision to the canonical cortical microcircuit.
Keywords: corticocortical neurons; corticothalamic neurons; fast-spiking interneurons; layer 6; neocortex; parvalbumin interneurons; thalamus.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures

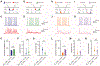

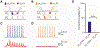
Similar articles
-
Specificity of synaptic connectivity between layer 1 inhibitory interneurons and layer 2/3 pyramidal neurons in the rat neocortex.Cereb Cortex. 2011 Aug;21(8):1818-26. doi: 10.1093/cercor/bhq257. Epub 2011 Jan 10. Cereb Cortex. 2011. PMID: 21220765 Free PMC article.
-
Neocortical inhibitory system.Folia Biol (Praha). 2009;55(6):201-17. Folia Biol (Praha). 2009. PMID: 20163769 Review.
-
Diverse types of interneurons generate thalamus-evoked feedforward inhibition in the mouse barrel cortex.J Neurosci. 2001 Apr 15;21(8):2699-710. doi: 10.1523/JNEUROSCI.21-08-02699.2001. J Neurosci. 2001. PMID: 11306623 Free PMC article.
-
Short-term dynamics of thalamocortical and intracortical synapses onto layer 6 neurons in neocortex.J Neurophysiol. 2002 Oct;88(4):1924-32. doi: 10.1152/jn.2002.88.4.1924. J Neurophysiol. 2002. PMID: 12364518
-
Interlaminar connections in the neocortex.Cereb Cortex. 2003 Jan;13(1):5-14. doi: 10.1093/cercor/13.1.5. Cereb Cortex. 2003. PMID: 12466210 Review.
Cited by
-
Form, synapses and orientation topography of a new cell type in layer 6 of the cat's primary visual cortex.Sci Rep. 2022 Sep 14;12(1):15428. doi: 10.1038/s41598-022-19746-9. Sci Rep. 2022. PMID: 36104476 Free PMC article.
-
Short-Term Facilitation of Long-Range Corticocortical Synapses Revealed by Selective Optical Stimulation.Cereb Cortex. 2022 Apr 20;32(9):1932-1949. doi: 10.1093/cercor/bhab325. Cereb Cortex. 2022. PMID: 34519352 Free PMC article.
-
Inhibitory neurons exhibit high controlling ability in the cortical microconnectome.PLoS Comput Biol. 2021 Apr 8;17(4):e1008846. doi: 10.1371/journal.pcbi.1008846. eCollection 2021 Apr. PLoS Comput Biol. 2021. PMID: 33831009 Free PMC article.
-
Network resonance and the auditory steady state response.Sci Rep. 2024 Jul 22;14(1):16799. doi: 10.1038/s41598-024-66697-4. Sci Rep. 2024. PMID: 39039107 Free PMC article.
-
Corticothalamic feedback sculpts visual spatial integration in mouse thalamus.Nat Neurosci. 2021 Dec;24(12):1711-1720. doi: 10.1038/s41593-021-00943-0. Epub 2021 Nov 11. Nat Neurosci. 2021. PMID: 34764474
References
-
- Agmon A, and Connors BW (1991). Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neuroscience 41, 365–379. - PubMed
-
- Arzt M, Sakmann B, and Meyer HS (2018). Anatomical correlates of local, translaminar, and transcolumnar inhibition by layer 6 GABAergic interneurons in somatosensory cortex. Cereb. Cortex 28, 2763–2774. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

