The human cytomegalovirus-encoded G protein-coupled receptor UL33 exhibits oncomodulatory properties
- PMID: 31519750
- PMCID: PMC6827316
- DOI: 10.1074/jbc.RA119.007796
The human cytomegalovirus-encoded G protein-coupled receptor UL33 exhibits oncomodulatory properties
Abstract
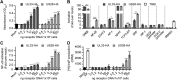
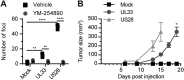
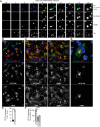
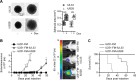
Herpesviruses can rewire cellular signaling in host cells by expressing viral G protein-coupled receptors (GPCRs). These viral receptors exhibit homology to human chemokine receptors, but some display constitutive activity and promiscuous G protein coupling. Human cytomegalovirus (HCMV) has been detected in multiple cancers, including glioblastoma, and its genome encodes four GPCRs. One of these receptors, US28, is expressed in glioblastoma and possesses constitutive activity and oncomodulatory properties. UL33, another HCMV-encoded GPCR, also displays constitutive signaling via Gαq, Gαi, and Gαs proteins. However, little is known about the nature and functional effects of UL33-driven signaling. Here, we assessed UL33's signaling repertoire and oncomodulatory potential. UL33 activated multiple proliferative, angiogenic, and inflammatory signaling pathways in HEK293T and U251 glioblastoma cells. Notably, upon infection, UL33 contributed to HCMV-mediated STAT3 activation. Moreover, UL33 increased spheroid growth in vitro and accelerated tumor growth in different in vivo tumor models, including an orthotopic glioblastoma xenograft model. UL33-mediated signaling was similar to that stimulated by US28; however, UL33-induced tumor growth was delayed. Additionally, the spatiotemporal expression of the two receptors only partially overlapped in HCMV-infected glioblastoma cells. In conclusion, our results unveil that UL33 has broad signaling capacity and provide mechanistic insight into its functional effects. UL33, like US28, exhibits oncomodulatory properties, elicited via constitutive activation of multiple signaling pathways. UL33 and US28 might contribute to HCMV's oncomodulatory effects through complementing and converging cellular signaling, and hence UL33 may represent a promising drug target in HCMV-associated malignancies.
Keywords: G protein-coupled receptor (GPCR); UL33; cell signaling; constitutive signaling; glioblastoma; herpesvirus; human cytomegalovirus (HCMV); oncogene.
© 2019 van Senten et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

Similar articles
-
Human Cytomegalovirus-Encoded G Protein-Coupled Receptor UL33 Facilitates Virus Dissemination via the Extracellular and Cell-to-Cell Route.Viruses. 2020 May 30;12(6):594. doi: 10.3390/v12060594. Viruses. 2020. PMID: 32486172 Free PMC article.
-
Constitutive Signaling by the Human Cytomegalovirus G Protein Coupled Receptor Homologs US28 and UL33 Enables Trophoblast Migration In Vitro.Viruses. 2022 Feb 14;14(2):391. doi: 10.3390/v14020391. Viruses. 2022. PMID: 35215985 Free PMC article.
-
Constitutive signaling of the human cytomegalovirus-encoded receptor UL33 differs from that of its rat cytomegalovirus homolog R33 by promiscuous activation of G proteins of the Gq, Gi, and Gs classes.J Biol Chem. 2003 Dec 12;278(50):50010-23. doi: 10.1074/jbc.M306530200. Epub 2003 Sep 30. J Biol Chem. 2003. PMID: 14522997
-
HCMV-encoded G-protein-coupled receptors as constitutively active modulators of cellular signaling networks.Trends Pharmacol Sci. 2006 Jan;27(1):56-63. doi: 10.1016/j.tips.2005.11.006. Epub 2005 Dec 13. Trends Pharmacol Sci. 2006. PMID: 16352349 Review.
-
Viral G protein-coupled receptors as modulators of cancer hallmarks.Pharmacol Res. 2020 Jun;156:104804. doi: 10.1016/j.phrs.2020.104804. Epub 2020 Apr 8. Pharmacol Res. 2020. PMID: 32278040 Review.
Cited by
-
Control of Cytokines in Latent Cytomegalovirus Infection.Pathogens. 2020 Oct 21;9(10):858. doi: 10.3390/pathogens9100858. Pathogens. 2020. PMID: 33096622 Free PMC article. Review.
-
Clinical implications of cytomegalovirus in glioblastoma progression and therapy.NPJ Precis Oncol. 2024 Sep 29;8(1):213. doi: 10.1038/s41698-024-00709-4. NPJ Precis Oncol. 2024. PMID: 39343770 Free PMC article. Review.
-
Patient-Oriented Perspective on Chemokine Receptor Expression and Function in Glioma.Cancers (Basel). 2021 Dec 28;14(1):130. doi: 10.3390/cancers14010130. Cancers (Basel). 2021. PMID: 35008294 Free PMC article. Review.
-
Human Cytomegalovirus-Encoded G Protein-Coupled Receptor UL33 Facilitates Virus Dissemination via the Extracellular and Cell-to-Cell Route.Viruses. 2020 May 30;12(6):594. doi: 10.3390/v12060594. Viruses. 2020. PMID: 32486172 Free PMC article.
-
Constitutive Signaling by the Human Cytomegalovirus G Protein Coupled Receptor Homologs US28 and UL33 Enables Trophoblast Migration In Vitro.Viruses. 2022 Feb 14;14(2):391. doi: 10.3390/v14020391. Viruses. 2022. PMID: 35215985 Free PMC article.
References
-
- Martin J. N. (2007) The epidemiology of KSHV and its association with malignant disease, in Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis (Arvin A., Campadelli-Fiume G., Mocarski E., Moore P. S., Roizman B., Whitley R., and Yamanishi K., eds) Chapter 54, Cambridge University Press, Cambridge, UK - PubMed
-
- Maussang D., Langemeijer E., Fitzsimons C. P., Stigter-van Walsum M., Dijkman R., Borg M. K., Slinger E., Schreiber A., Michel D., Tensen C. P., van Dongen G. A., Leurs R., and Smit M. J. (2009) The human cytomegalovirus-encoded chemokine receptor US28 promotes angiogenesis and tumor formation via cyclooxygenase-2. Cancer Res. 69, 2861–2869 10.1158/0008-5472.CAN-08-2487 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

