Early Nuclear Events after Herpesviral Infection
- PMID: 31500286
- PMCID: PMC6780142
- DOI: 10.3390/jcm8091408
Early Nuclear Events after Herpesviral Infection
Abstract
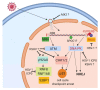
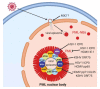
Herpesviruses are important pathogens that can cause significant morbidity and mortality in the human population. Herpesviruses have a double-stranded DNA genome, and viral genome replication takes place inside the nucleus. Upon entering the nucleus, herpesviruses have to overcome the obstacle of cellular proteins in order to enable viral gene expression and genome replication. In this review, we want to highlight cellular proteins that sense incoming viral genomes of the DNA-damage repair (DDR) pathway and of PML-nuclear bodies (PML-NBs) that all can act as antiviral restriction factors within the first hours after the viral genome is released into the nucleus. We show the function and significance of both nuclear DNA sensors, the DDR and PML-NBs, and demonstrate for three human herpesviruses of the alpha-, beta- and gamma-subfamilies, HSV-1, HCMV and KSHV respectively, how viral tegument proteins antagonize these pathways.
Keywords: ATRX; CMV; DAXX; DNA-damage repair; DNA-damage response; HHV-6; HR; HSV-1; Herpes simplex virus; Herpesviruses; ICP-0; IE-1; KSHV; Kaposi’s sarcoma-associated herpesvirus; ND10; NHEJ; ORF75; PML; PML nuclear bodies; SP100; cytomegalovirus; double strand break; double-stranded DNA virus; homology repair; infection; non-homologous end joining; nuclear DNA sensors; restriction factors; virus; virus-host interaction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Kaposi's sarcoma associated herpesvirus tegument protein ORF75 is essential for viral lytic replication and plays a critical role in the antagonization of ND10-instituted intrinsic immunity.PLoS Pathog. 2014 Jan;10(1):e1003863. doi: 10.1371/journal.ppat.1003863. Epub 2014 Jan 16. PLoS Pathog. 2014. PMID: 24453968 Free PMC article.
-
Viral FGARAT Homolog ORF75 of Rhesus Monkey Rhadinovirus Effects Proteasomal Degradation of the ND10 Components SP100 and PML.J Virol. 2016 Aug 12;90(17):8013-28. doi: 10.1128/JVI.01181-16. Print 2016 Sep 1. J Virol. 2016. PMID: 27356898 Free PMC article.
-
Mechanisms of Host IFI16, PML, and Daxx Protein Restriction of Herpes Simplex Virus 1 Replication.J Virol. 2018 Apr 27;92(10):e00057-18. doi: 10.1128/JVI.00057-18. Print 2018 May 15. J Virol. 2018. PMID: 29491153 Free PMC article.
-
Gammaherpesviral Tegument Proteins, PML-Nuclear Bodies and the Ubiquitin-Proteasome System.Viruses. 2017 Oct 21;9(10):308. doi: 10.3390/v9100308. Viruses. 2017. PMID: 29065450 Free PMC article. Review.
-
The Role of ND10 Nuclear Bodies in Herpesvirus Infection: A Frenemy for the Virus?Viruses. 2021 Feb 3;13(2):239. doi: 10.3390/v13020239. Viruses. 2021. PMID: 33546431 Free PMC article. Review.
Cited by
-
Epigenetic Restriction Factors (eRFs) in Virus Infection.Viruses. 2024 Jan 25;16(2):183. doi: 10.3390/v16020183. Viruses. 2024. PMID: 38399958 Free PMC article. Review.
-
Bright and Early: Inhibiting Human Cytomegalovirus by Targeting Major Immediate-Early Gene Expression or Protein Function.Viruses. 2020 Jan 16;12(1):110. doi: 10.3390/v12010110. Viruses. 2020. PMID: 31963209 Free PMC article. Review.
-
Functional regulation of the structure-specific endonuclease FEN1 by the human cytomegalovirus protein IE1 suggests a role for the re-initiation of stalled viral replication forks.PLoS Pathog. 2021 Mar 26;17(3):e1009460. doi: 10.1371/journal.ppat.1009460. eCollection 2021 Mar. PLoS Pathog. 2021. PMID: 33770148 Free PMC article.
-
Intrinsic Immune Mechanisms Restricting Human Cytomegalovirus Replication.Viruses. 2021 Jan 26;13(2):179. doi: 10.3390/v13020179. Viruses. 2021. PMID: 33530304 Free PMC article. Review.
-
Immune Response to Herpes Simplex Virus Infection and Vaccine Development.Vaccines (Basel). 2020 Jun 12;8(2):302. doi: 10.3390/vaccines8020302. Vaccines (Basel). 2020. PMID: 32545507 Free PMC article. Review.
References
-
- Pellet P.E., Roizman B. Fields Virology. Wolters Kluwer; Alfon am Rhein, The Netherlands: 2013.
-
- Emery V.C., Clark D.A. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge University Press; Cambridge, UK: 2007. HHV-6A, 6B, and 7: Persistence in the population, epidemiology and transmission. - PubMed
-
- Hjalgrim H., Friborg J., Melbye M. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge University Press; Cambridge, UK: 2007. The epidemiology of EBV and its association with malignant disease. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

