Lactoferrin CpG Island Hypermethylation and Decoupling of mRNA and Protein Expression in the Early Stages of Prostate Carcinogenesis
- PMID: 31499027
- PMCID: PMC6892185
- DOI: 10.1016/j.ajpath.2019.07.016
Lactoferrin CpG Island Hypermethylation and Decoupling of mRNA and Protein Expression in the Early Stages of Prostate Carcinogenesis
Abstract
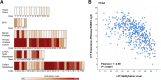
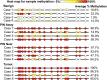
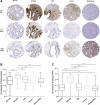
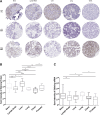
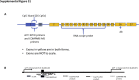
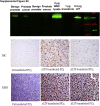
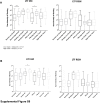
Lactoferrin (LTF) is an iron-binding protein canonically known for its innate and adaptive immune functions. LTF may also act as a tumor suppressor with antiproliferative action. LTF is inactivated genetically or epigenetically in various cancers, and a CpG island spanning the transcriptional start site of LTF is hypermethylated in prostate cancer cell lines. We, therefore, hypothesized that LTF expression is silenced via CpG island hypermethylation in the early stages of prostate tumorigenesis carcinogenesis. Targeted methylation analysis was performed using a combination of methylated-DNA precipitation and methylation-sensitive restriction enzymes, and laser-capture microdissection followed by bisulfite sequencing on DNA isolated from prostate tissue samples, including both primary and metastatic disease. LTF mRNA in situ hybridization and LTF protein immunohistochemistry were also performed. We report that the LTF CpG island is frequently and densely methylated in high-grade prostatic intraepithelial neoplasia, primary prostate carcinoma, and metastases. We further report a decoupling of lactoferrin mRNA and protein expression, including in lesions where LTF mRNA has presumably been silenced via CpG island methylation. We conclude that LTF mRNA expression is silenced in prostate tumorigenesis via hypermethylation, supporting a role for LTF as a prostate cancer tumor suppressor gene. Likewise, the frequency at which the LTF CpG island is methylated across samples suggests it is an important and conserved step in prostate cancer initiation.
Copyright © 2019 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures








Similar articles
-
Aberrant CpG island hypermethylation of multiple genes in prostate cancer and prostatic intraepithelial neoplasia.J Pathol. 2004 Feb;202(2):233-40. doi: 10.1002/path.1503. J Pathol. 2004. PMID: 14743506
-
Genomic hypomethylation and CpG island hypermethylation in prostatic intraepithelial neoplasm.Virchows Arch. 2009 Jan;454(1):17-23. doi: 10.1007/s00428-008-0706-6. Epub 2008 Dec 2. Virchows Arch. 2009. PMID: 19048291
-
DNA methylation paradigm shift: 15-lipoxygenase-1 upregulation in prostatic intraepithelial neoplasia and prostate cancer by atypical promoter hypermethylation.Prostaglandins Other Lipid Mediat. 2007 Jan;82(1-4):185-97. doi: 10.1016/j.prostaglandins.2006.05.015. Epub 2006 Jul 11. Prostaglandins Other Lipid Mediat. 2007. PMID: 17164146
-
GSTP1 CpG island hypermethylation as a molecular biomarker for prostate cancer.J Cell Biochem. 2004 Feb 15;91(3):540-52. doi: 10.1002/jcb.10740. J Cell Biochem. 2004. PMID: 14755684 Review.
-
[GSTP1 CpG island hypermethylation as a molecular marker of prostate cancer].Urologe A. 2004 May;43(5):573-9. doi: 10.1007/s00120-004-0540-7. Urologe A. 2004. PMID: 15029477 Review. German.
Cited by
-
Single Nucleotide Polymorphism and mRNA Expression of LTF in Oral Squamous Cell Carcinoma.Genes (Basel). 2022 Nov 10;13(11):2085. doi: 10.3390/genes13112085. Genes (Basel). 2022. PMID: 36360322 Free PMC article.
-
Expression Profiles of CDKN2A, MDM2, E2F2 and LTF Genes in Oral Squamous Cell Carcinoma.Biomedicines. 2022 Nov 23;10(12):3011. doi: 10.3390/biomedicines10123011. Biomedicines. 2022. PMID: 36551770 Free PMC article.
-
Club-like cells in proliferative inflammatory atrophy of the prostate.J Pathol. 2023 Sep;261(1):85-95. doi: 10.1002/path.6149. Epub 2023 Aug 7. J Pathol. 2023. PMID: 37550827 Free PMC article.
-
Convergent alterations in the tumor microenvironment of MYC-driven human and murine prostate cancer.Nat Commun. 2024 Aug 28;15(1):7414. doi: 10.1038/s41467-024-51450-2. Nat Commun. 2024. PMID: 39198404 Free PMC article.
-
Health inequity drives disease biology to create disparities in prostate cancer outcomes.J Clin Invest. 2022 Feb 1;132(3):e155031. doi: 10.1172/JCI155031. J Clin Invest. 2022. PMID: 35104804 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

