Regulation of active and quiescent somatic stem cells by Notch signaling
- PMID: 31489617
- PMCID: PMC7027910
- DOI: 10.1111/dgd.12626
Regulation of active and quiescent somatic stem cells by Notch signaling
Abstract
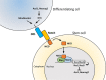
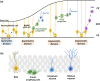
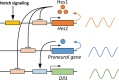
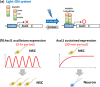
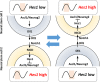
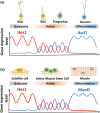
Somatic stem/progenitor cells actively proliferate and give rise to different types of mature cells (active state) in embryonic tissues while they are mostly dormant (quiescent state) in many adult tissues. Notch signaling is known to regulate both active and quiescent states of somatic stem cells, but how it regulates these different states is unknown. Recent studies revealed that the Notch effector Hes1 is expressed differently during the active and quiescent states during neurogenesis and myogenesis: high in the quiescent state and oscillatory in the active state. When the Hes1 expression level is high, both Ascl1 and MyoD expression are continuously suppressed. By contrast, when Hes1 expression oscillates, it periodically represses expression of the neurogenic factor Ascl1 and the myogenic factor MyoD, thereby driving Ascl1 and MyoD oscillations. High levels of Hes1 and the resultant Ascl1 suppression promote the quiescent state of neural stem cells, while Hes1 oscillation-dependent Ascl1 oscillations regulate their active state. Similarly, in satellite cells of muscles, known adult muscle stem cells, high levels of Hes1 and the resultant MyoD suppression seem to promote their quiescent state, while Hes1 oscillation-dependent MyoD oscillations activate their proliferation and differentiation. Therefore, the expression dynamics of Hes1 is a key regulatory mechanism of generating and maintaining active/quiescent stem cell states.
Keywords: Hes1; Notch signaling; active stem cell; oscillatory expression; quiescent stem cell.
© 2019 The Authors. Development, Growth & Differentiation published by John Wiley & Sons Australia, Ltd on behalf of Japanese Society of Developmental Biologists.
Figures
Similar articles
-
High Hes1 expression and resultant Ascl1 suppression regulate quiescent vs. active neural stem cells in the adult mouse brain.Genes Dev. 2019 May 1;33(9-10):511-523. doi: 10.1101/gad.323196.118. Epub 2019 Mar 12. Genes Dev. 2019. PMID: 30862661 Free PMC article.
-
The significance of gene expression dynamics in neural stem cell regulation.Proc Jpn Acad Ser B Phys Biol Sci. 2020;96(8):351-363. doi: 10.2183/pjab.96.026. Proc Jpn Acad Ser B Phys Biol Sci. 2020. PMID: 33041269 Free PMC article. Review.
-
Hes1 oscillation frequency correlates with activation of neural stem cells.Gene Expr Patterns. 2021 Jun;40:119170. doi: 10.1016/j.gep.2021.119170. Epub 2021 Mar 3. Gene Expr Patterns. 2021. PMID: 33675998
-
An oscillatory network controlling self-renewal of skeletal muscle stem cells.Exp Cell Res. 2021 Dec 15;409(2):112933. doi: 10.1016/j.yexcr.2021.112933. Epub 2021 Nov 15. Exp Cell Res. 2021. PMID: 34793773 Review.
-
Ultradian oscillations in Notch signaling regulate dynamic biological events.Curr Top Dev Biol. 2010;92:311-31. doi: 10.1016/S0070-2153(10)92010-3. Curr Top Dev Biol. 2010. PMID: 20816400 Review.
Cited by
-
Engineering tissue morphogenesis: taking it up a Notch.Trends Biotechnol. 2022 Aug;40(8):945-957. doi: 10.1016/j.tibtech.2022.01.007. Epub 2022 Feb 15. Trends Biotechnol. 2022. PMID: 35181146 Free PMC article. Review.
-
Overview of Three Proliferation Pathways (Wnt, Notch, and Hippo) in Intestine and Immune System and Their Role in Inflammatory Bowel Diseases (IBDs).Front Med (Lausanne). 2022 May 23;9:865131. doi: 10.3389/fmed.2022.865131. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35677821 Free PMC article. Review.
-
Lineage Reprogramming: Genetic, Chemical, and Physical Cues for Cell Fate Conversion with a Focus on Neuronal Direct Reprogramming and Pluripotency Reprogramming.Cells. 2024 Apr 19;13(8):707. doi: 10.3390/cells13080707. Cells. 2024. PMID: 38667322 Free PMC article. Review.
-
LncRNA RUS shapes the gene expression program towards neurogenesis.Life Sci Alliance. 2022 Jun 10;5(10):e202201504. doi: 10.26508/lsa.202201504. Print 2022 Oct. Life Sci Alliance. 2022. PMID: 35688487 Free PMC article.
-
A review on Tsukushi: mammalian development, disorders, and therapy.J Cell Commun Signal. 2022 Dec;16(4):505-513. doi: 10.1007/s12079-022-00669-z. Epub 2022 Mar 1. J Cell Commun Signal. 2022. PMID: 35233735 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

